Roche Diagnostics
Instructions for Use · Version 13.0 207
cobas b 123 POC system 7 Quality control
Configuring QC rules and consequences
Configuring QC rules and consequences
WARNING
Failure to follow QC protocols or ignoring QC results may lead to incorrect patient
results
Failure to follow QC protocols or ignoring QC results may lead to incorrect patient results,
which may endanger patient lives.
r Follow quality control practices according to local regulations.
r Perform a minimum of one QC measurement each day. In addition, alternate through
the 3 levels of available QC materials over the course of 3 days. For example, perform a
QC measurement on day 1 with a level 1 QC material, on day 2 with a level 2 QC
material and on day 3 with a level 3 QC material.
r Perform QC tests on 3 levels after each of these actions: Fluid Pack and
Sensor Cartridge replacement, and installing and turning on the instrument.
r If QC results do not match their expected results, perform the QC measurements again.
If QC results still do not match their expected results, refer to the QC troubleshooting
section (QC troubleshooting (p. 218)). If the error persists, contact your Roche Service
representative.
r Do not use the system for diagnostic purposes until QC results match their expected
results.
To ensure that there is a QC consequence when the rules for QC evaluation are
broken, you should assign all parameters to the QC consequence “QC lock”.
p To define QC rules and consequences for a parameter
1
Utilities > Configuration > Quality control > Rules / conseq
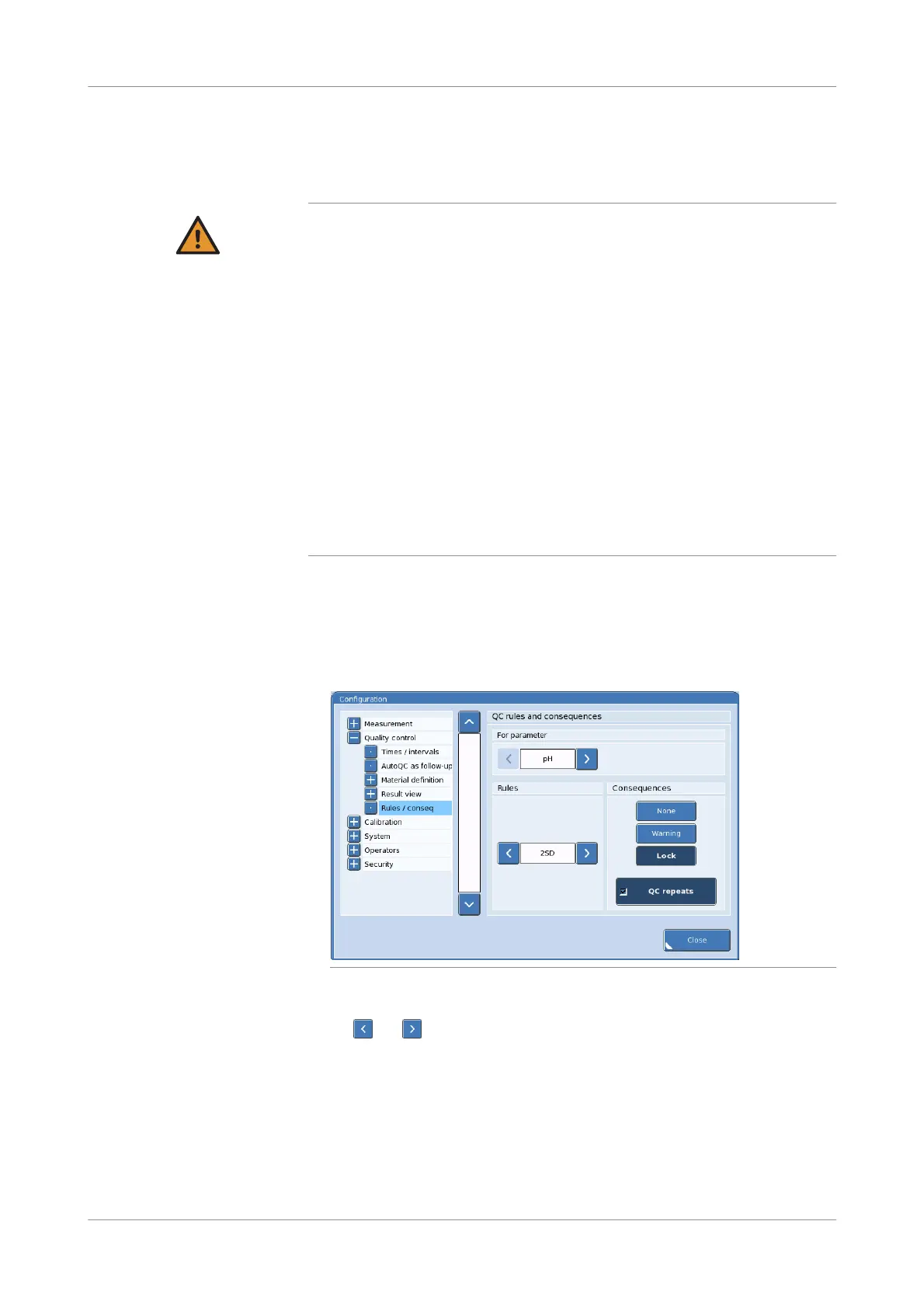
Figure 7-38 Rules and consequences
2
To select a parameter to define QC rules and consequences for, press
the and buttons at the top of the QC rules and consequences panel.
 Loading...
Loading...