Roche Diagnostics
Instructions for Use · Version 13.0 281
cobas b 123 POC system 10 Software functions
Configuring operator settings
p To delete an operator
1
Utilities > Configuration > Operators
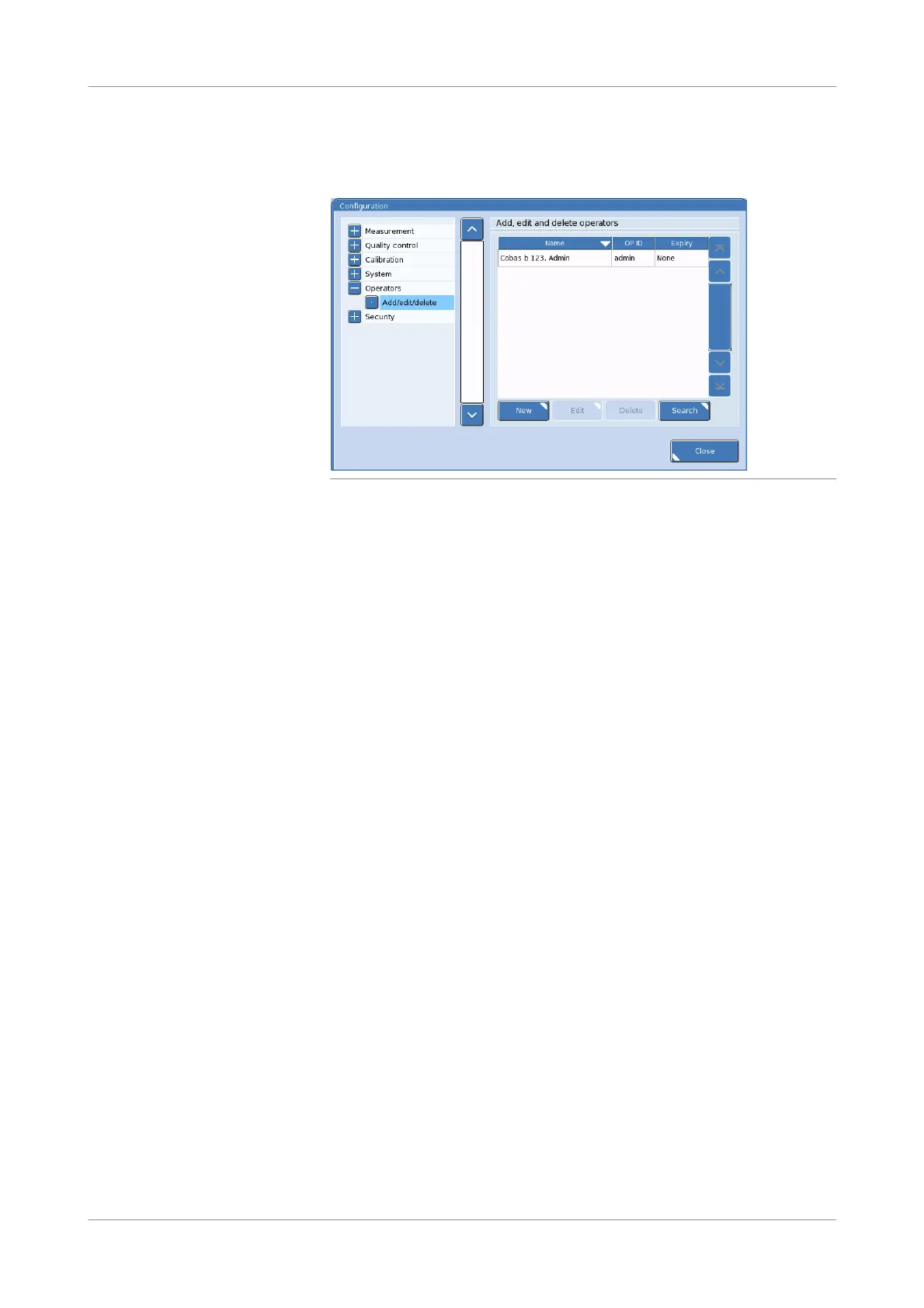
Figure 10-43 Operator settings screen
2
Select the operator that you want to delete. Then, press the Delete button.
s
 Loading...
Loading...