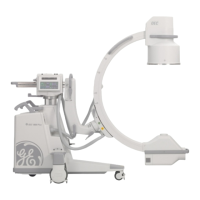
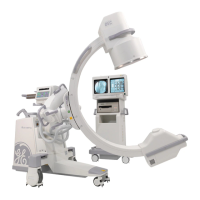
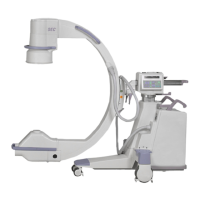
Brivo OEC 715/785/865 Mobile C-Arm X-Ray Product Service Manual
2
REGULATORY REQUIREMENTS
International Electrotechnical Commission (IEC), international standards organization, when
applicable.
Brivo OEC 715/Brivo OEC 785/Brivo OEC 865 complies with the regulatory requirements of the
following:
Council Directive 93/42/EEC concerning medical devices.
CE label affixed to the product testifies compliance to all requirements of the Directive.
The location of the CE
0459
label is described in this manual.
European Representative:
GE Medical Systems S.C.S.
Quality Assurance Manager
283 rue de la Minière
78530 BUC France
Tel: +33 1 30 70 40 40
 Loading...
Loading...