Chapter 2: Theory of Operation 51
Fluorescent Signals
When cells or particles stained with fluorochrome-conjugated antibodies or other
dyes pass through a laser beam, the dyes can absorb photons (energy) and be
promoted to an excited electronic state. In returning to their ground state, the
dyes release energy, most of which is emitted as light. This light emission is
known as fluorescence.
Fluorescence is always a longer wavelength (lower-energy photon) than the
excitation wavelength. The difference between the excitation wavelength and the
emission wavelength is known as the Stokes shift. Some fluorescent compounds
such as PerCP exhibit a large Stokes shift, absorbing blue light (488 nm) and
emitting red light (675 nm), while other fluorochromes such as FITC have a
smaller Stokes shift, absorbing blue light and emitting green light (530 nm).
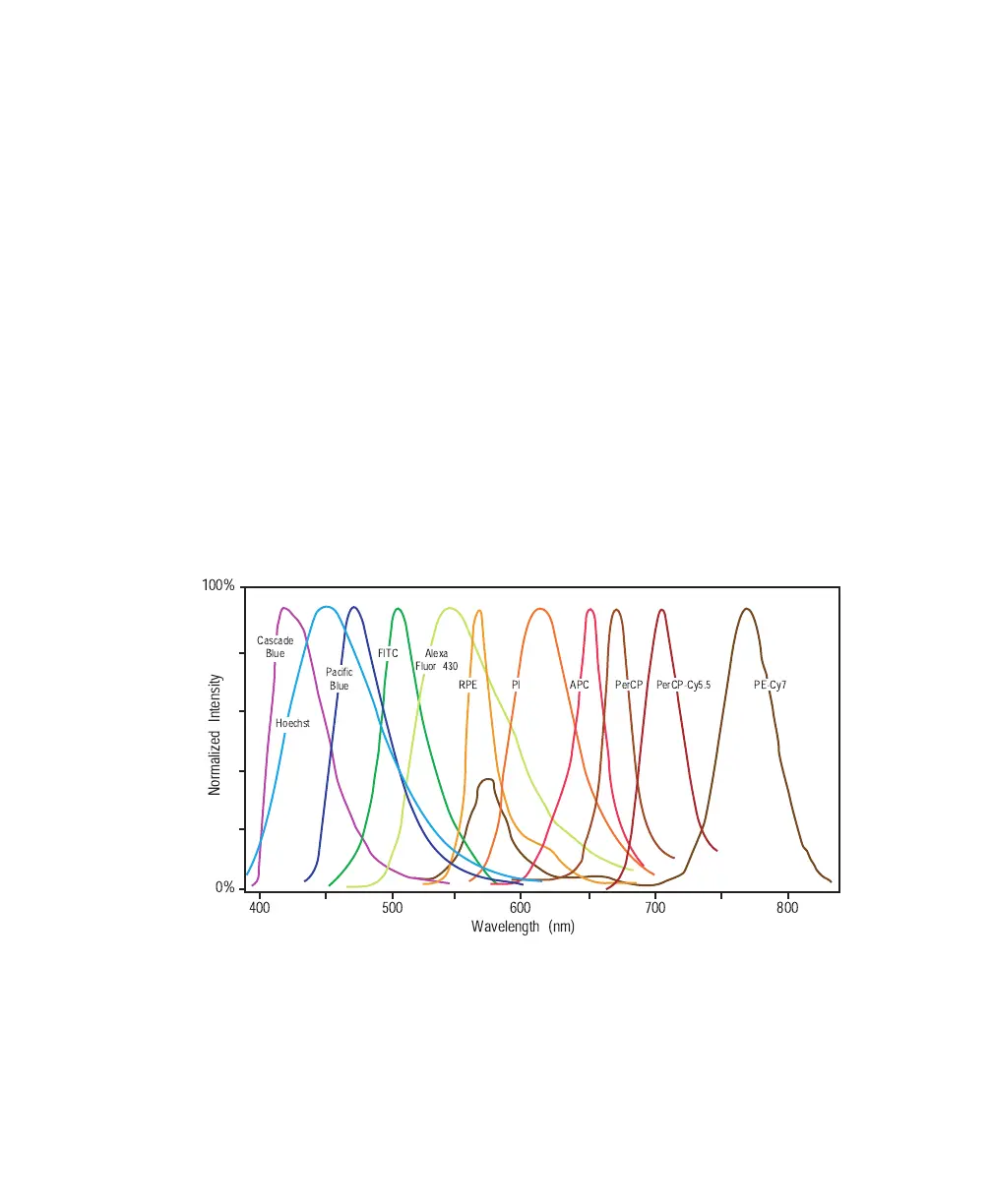
The emission spectra for some commonly used fluorochromes are shown in
Figure 2-5. See Table 1-1 on page 40 for the corresponding detectors.
Figure 2-5 Emission spectra of commonly used fluorochromes
400
0%
100%
500
Wavelength (nm)
Normalized Intensity
600 700 800
Cascade
Blue
FITC
RPE
PI
APC PerCP PerCP-Cy5.5
PE-Cy7
Pacific
Blue
Hoechst
Alexa
Fluor 430
 Loading...
Loading...