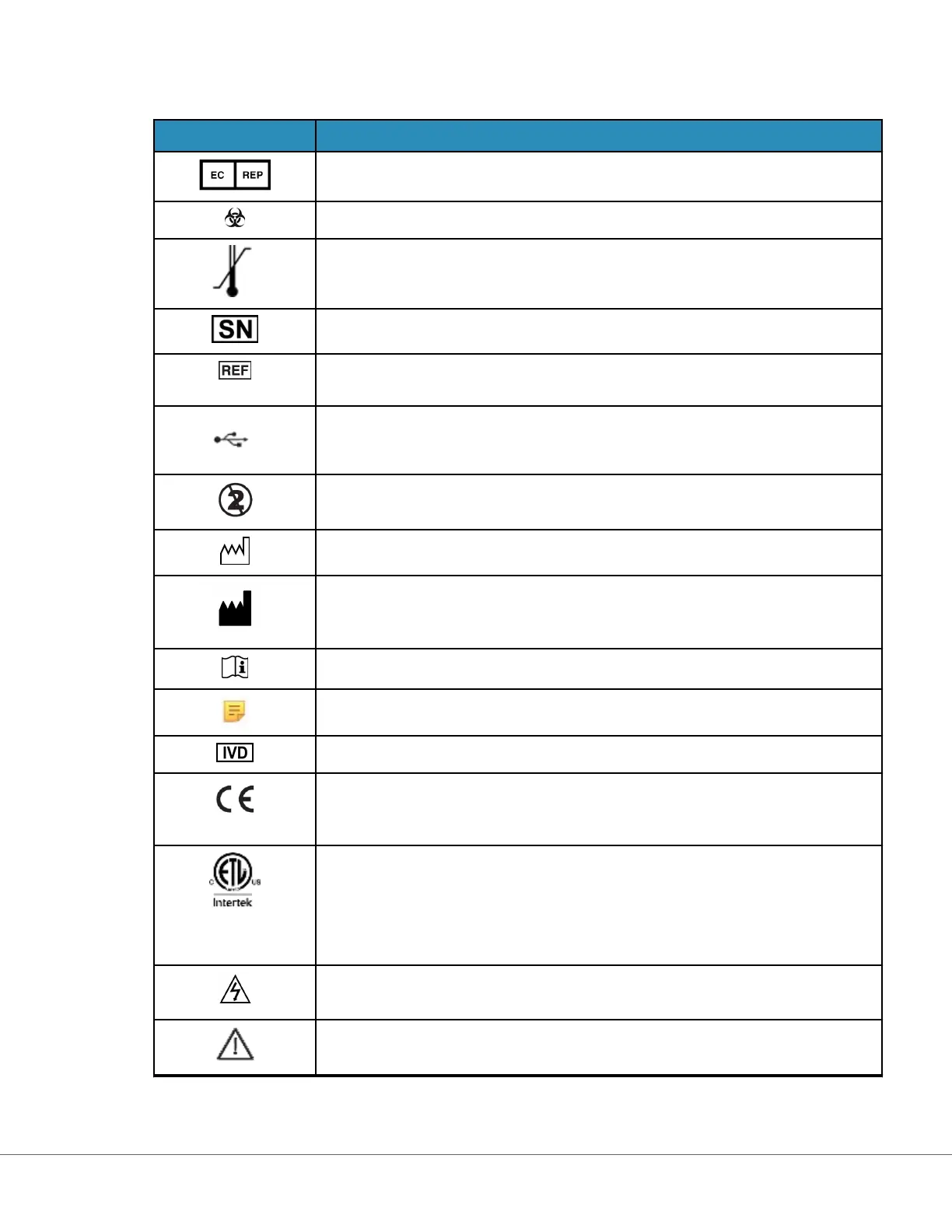
Table 5: Regulatory and Safety Related; Miscellaneous
Symbol Denion/Use
Authorized representave for Regulatory Aairs in the European Community.
Biological risks
Temperature limitaons. The upper and lower limits for storage are adjacent to
upper and lower arms.
Serial number. The serial number will appear adjacent to this symbol.
Catalog number, list number, or reference number. The number adjacent to this
symbol is used to reorder the product.
USB
Do not reuse.
Date of manufacture
Manufacturer
Consult instrucons for use or see System Operaons Manual for instrucons.
Note the following informaon.
In vitro diagnosc medical device
A mark that indicates conformity to the legal requirements of the appropriate
European Union (EU) Direcve(s) with respect to safety, health, environment
and consumer protecon.
Signies that the product bearingt he ETL Listed mark complies with both U.S.
and Canadian product safety standards:
UL 61010-1: 3rd Ed; Am.1
CAN/CSA C22.2 No. 61010-1-12 3rd Ed. (R2017) +U1;U2
Electrical hazard
Aenon: See instrucons for use.
xxviii
i-STAT Alinity — System Operaons Manual Art: 745579-01 Rev. K Rev. Date: 16-May-2022
 Loading...
Loading...