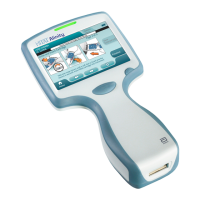
1.1 - Complete i‑STAT Alinity System Overview
The i‑STAT Alinity instrument is an analycal, in vitro diagnosc device. The instrument requires i‑STAT
single-use cartridges containing electrodes and sensors to perform quantave diagnosc tesng on
whole blood or plasma. Together, the instrument and cartridge allow the user to perform clinical tesng
and related administrave tasks.
The i‑STAT Alinity design enables the instrument to be taken to the paent’s bedside (point of care), a
convenient locaon near the point of care, or clinical laboratory seng.
Aer the inseron of a lled test cartridge, the instrument carefully monitors and controls the tesng
process. The only user intervenon is in the form of data entry, performed via the touchscreen or by
barcode capture. Throughout the cycle, the instrument performs a series of quality checks. These checks
are designed to monitor the status of the instrument and the quality of the cartridge. An i‑STAT Alinity
instrument, a cartridge with the required test, and two or three drops of blood will allow the caregiver to
view quantave test results within minutes.
For the purposes of this system operaons manual, the i‑STAT Alinity system components and associated
accessories will be discussed. Addional informaon related to the enre system can be found in the
i‑STAT Alinity system documentaon listed below.
Note: Note Regarding System Reliability: The i-STAT System automacally runs a
comprehensive set of quality checks of analyzer and cartridge performance each me a
sample is tested. This internal quality system will suppress results if the analyzer or
cartridge does not meet certain internal specicaons (see Quality Control secon in
System Operaons Manual for detailed informaon). To minimize the probabilty of
delivering a result with medically signicant error, the internal specicaons are very
stringent. It is typical for the system to suppress a very small percentage of results in
normal operaon given the stringency of these specicaons. If however, the analyzer or
cartridges have been compromised, results may be persistently suppressed, and one or
the other must be replaced to restore normal operang condions. Where unavailability
of results while awaing replacement of analyzers or cartridges is unacceptable, APOC
recommends maintaining both a back up i-STAT System instrument and cartridges from
an alternate lot number.
i
‑STAT Alinity Documentaon:
• i‑STAT Alinity System Operaons Manual, including:
○ i‑STAT Alinity Reference
○ i-STAT Cartridge Instrucons for Use
○ AlinIQ NCi - Network Connecvity for i‑STAT Alinity
○ AlinIQ CWi - Customizaon Workspace for i‑STAT Alinity
• i‑STAT Alinity Quick Reference Guide
• i‑STAT Alinity Geng Started Guides:
○ i‑STAT Alinity Base Staon
○ i‑STAT Alinity Rechargeable Baery
○ i‑STAT Alinity Electronic Simulator
○ i‑STAT Alinity Printer
3
i-STAT Alinity — System Operaons Manual Art: 745526-01 Rev. H Rev. Date: 04-Mar-2021

 Loading...
Loading...