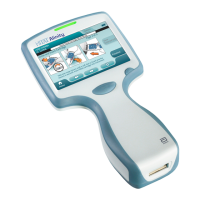
1.2 - i-STAT Alinity Instrument
Intended Use
The i‑STAT Alinity instrument is intended for use in the in vitro quancaon of various analytes in whole
blood or plasma in point of care or clinical laboratory sengs.
Instrument and cartridges should be used by healthcare professionals trained and cered to use the
system and should be used according to the facility's policies and procedures.
Note: Consult the IFU/CTI for details on specic sample types for the cartridge.
For in vitro diagnosc use.
Note: Not all cartridges are available in all regions. Check with your local representave
for availability in specic markets.
Note: To congure the instrument's prinng method, refer to the i-STAT Alinity Printer
Principles of Operaon
Verify the instrument for cartridge tesng
Note: Vericaon is only required once per cartridge type per instrument.
Prior to using an instrument requiring a specic cartridge type, verify the instrument supports the
cartridge:
1. Iniate a liquid quality control test per the instrucons in Liquid Quality Controls of the System
Operaons Manual.
2. Ensure the instrument can successfully scan the cartridge pouch barcode.
3. If the cartridge is not recognized, contact your local representave.
7
i-STAT Alinity — System Operaons Manual Art: 745527-01 Rev. N Rev. Date: 13-Oct-2022
 Loading...
Loading...