B-12
Theory of Operation
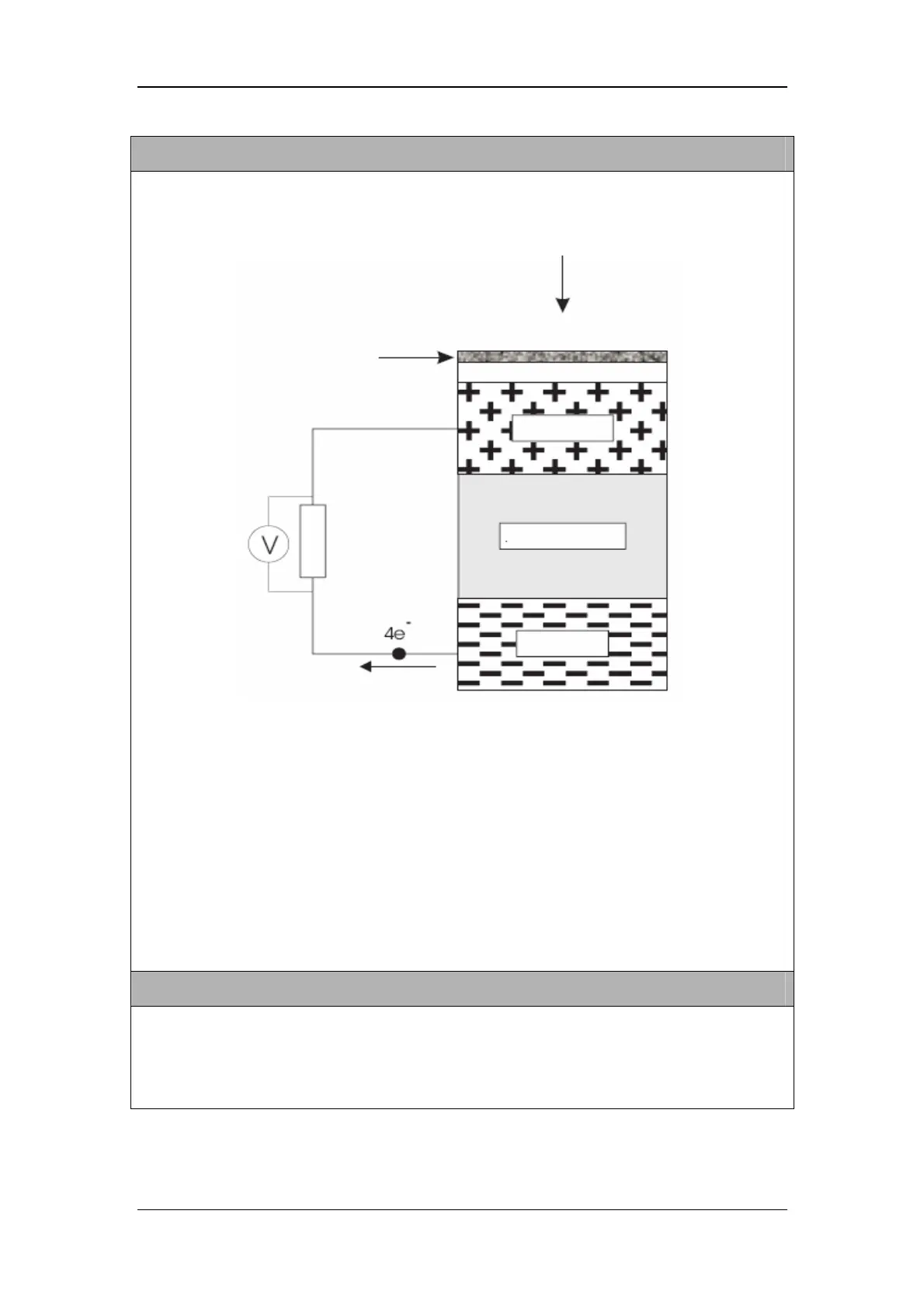
O2 sensor can monitor the patient’s FiO2. O2 sensor is of the self-powered, diffusion limited,
metal-air battery type comprising an anode, electrolyte, diffusion barrier and air cathode as shown
below:
At the cathode oxygen is reduced to hydroxyl ions according to the equation:
O2 + 2H20 + 4e- → 4OH
The hydroxyl ions in turn oxidise the metal anode as follows:
2Pb + 4OH- → 2PbO + 2H2O + 4e-
Overall the cell reaction may be represented as:
2Pb + O2 → 2PbO
O2 sensor is current generator, and the current is proportional to the rate of oxygen consumption
(Faraday's Law). This current can be measured by connecting a resistor across the output terminals
to produce a voltage signal. If the passage of oxygen into the sensor is purely diffusion limited, by
the solid membrane diffusion barrier, then this signal is a measure of the oxygen partial pressure.
Signal Stability
O2 sensor has highly stable outputs over their operating lives. Typical sensor drift rates are less
than 1% per month when O2 sensor is exposed to gas in typical applications. Thus a sensor with a
starting signal of 12mV in 210mBar oxygen will typically still be showing a signal greater than
10mV as it approaches the end of its life.
Air supply
Cathode
Electrolyte
Anode
Load resistor
Solid membrane
Diffusion barrier

 Loading...
Loading...