242 1100 Series FD Reference Manual
7 Introduction to the Fluorescence Detector
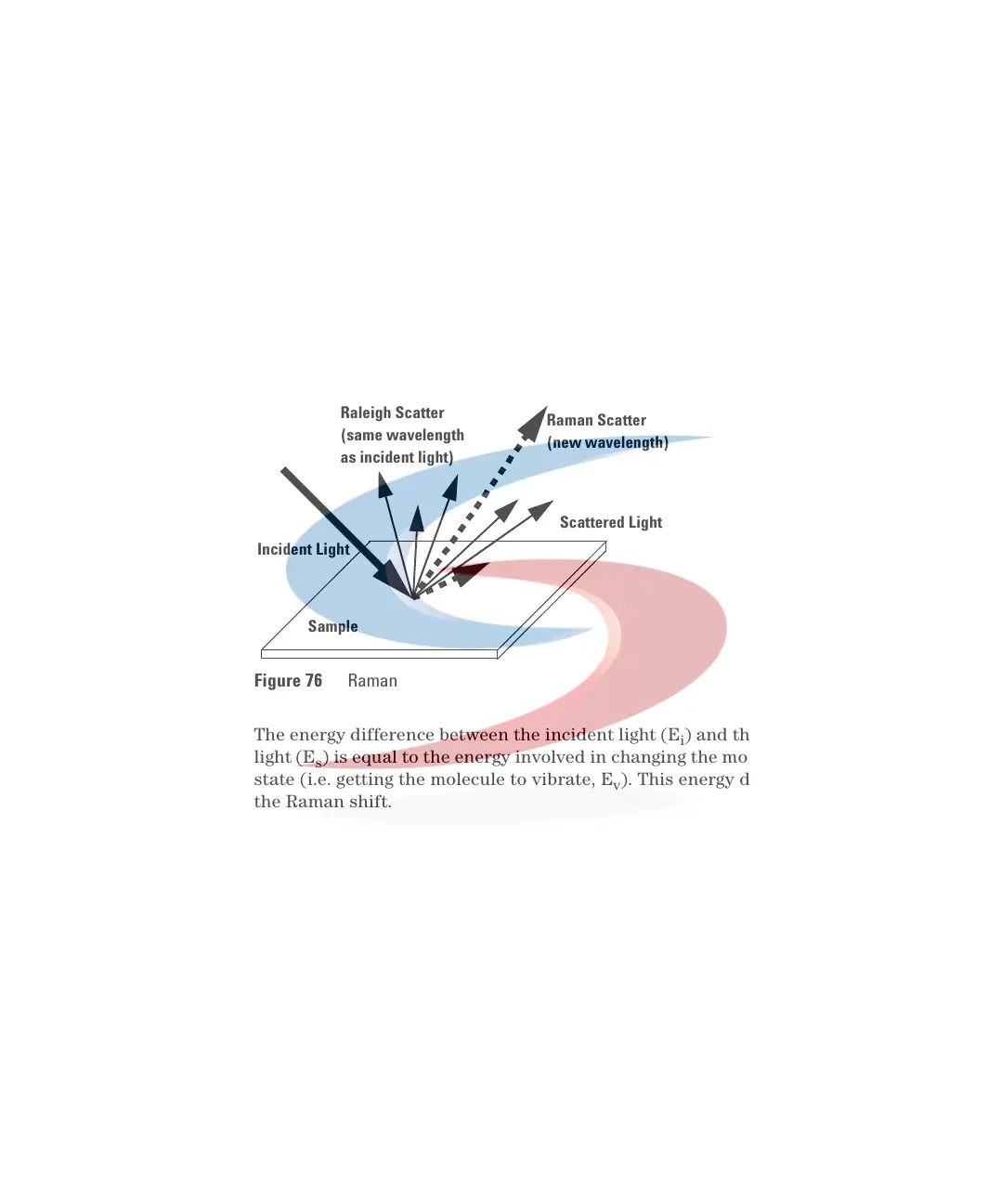
Raman Effect
The Raman effect arises when the incident light excites molecules in the
sample which subsequently scatter the light. While most of this scattered light
is at the same wavelength as the incident light, some is scattered at a different
wavelength. This inelastically scattered light is called Raman scatter. It results
from the molecule changing it's molecular motions.
The energy difference between the incident light (E
i
) and the Raman scattered
light (E
s
) is equal to the energy involved in changing the molecule's vibrational
state (i.e. getting the molecule to vibrate, E
v
). This energy difference is called
the Raman shift.
E
v
= E
i
- E
s
Several different Raman shifted signals will often be observed; each being
associated with different vibrational or rotational motions of molecules in the
sample. The particular molecule and its environment will determine what
Raman signals will be observed (if any).
A plot of Raman intensity versus Raman shift is a Raman spectrum.
Figure 76 Raman
Raman Scatter
(new wavelength)
Raleigh Scatter
(same wavelength
as incident light)
Sample
Incident Light
Scattered Light
 Loading...
Loading...
















