Introduction to the Fluorescence Detector 7
1100 Series FD Reference Manual 241
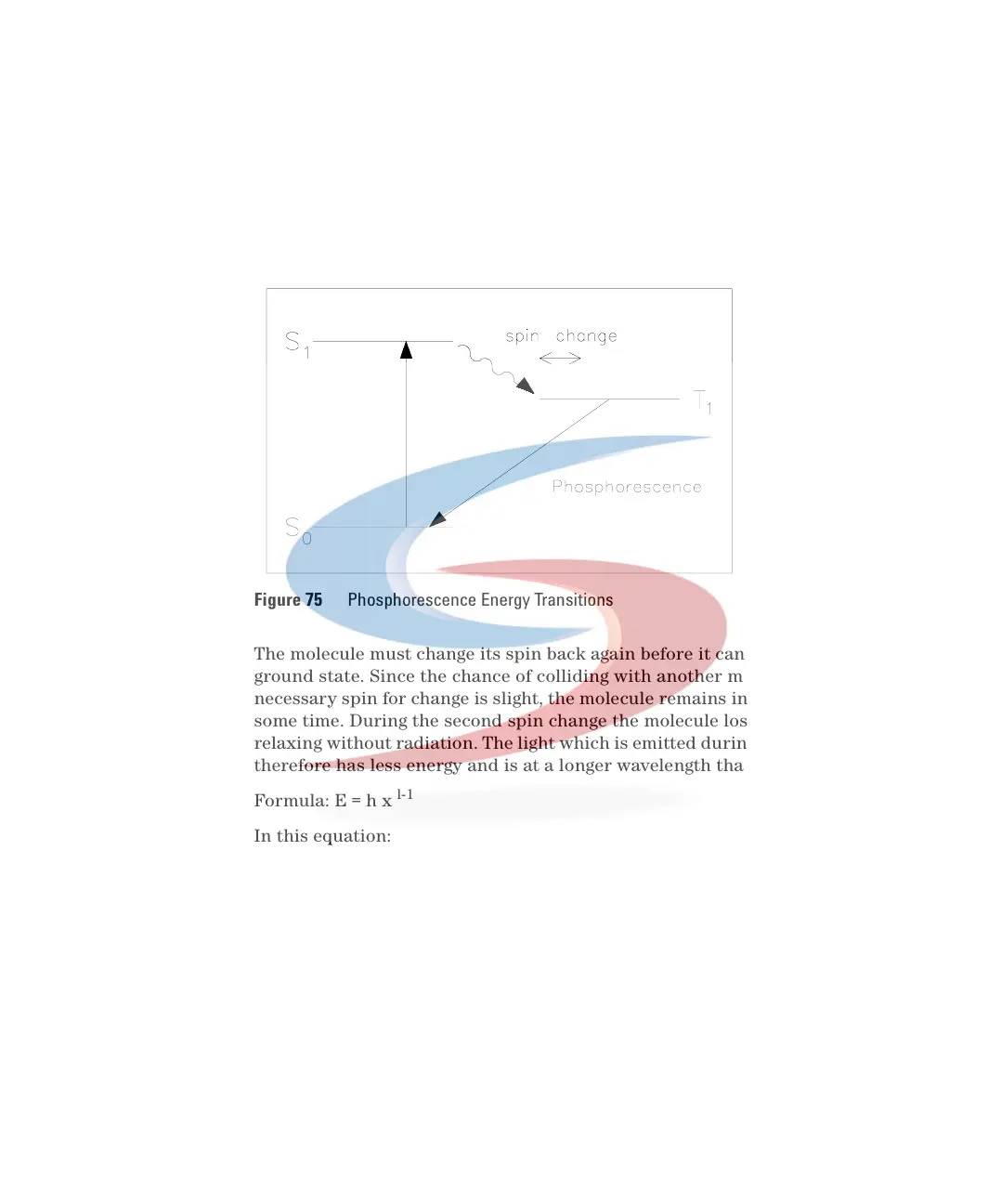
Phosphorescence is a longer process because one of the electrons involved in
the excitation changes its spin, during a collision with a molecule of solvent,
for example. The excited molecule is now in a so-called triplet state, T, see
Figure 75.
The molecule must change its spin back again before it can return to its
ground state. Since the chance of colliding with another molecule with the
necessary spin for change is slight, the molecule remains in its triplet state for
some time. During the second spin change the molecule loses more energy by
relaxing without radiation. The light which is emitted during phosphorescence
therefore has less energy and is at a longer wavelength than fluorescence.
Formula: E = h x
l-1
In this equation:
E is energy
h is Planck's constant
l
is the wavelength
Figure 75 Phosphorescence Energy Transitions
 Loading...
Loading...
















