240 1100 Series FD Reference Manual
7 Introduction to the Fluorescence Detector
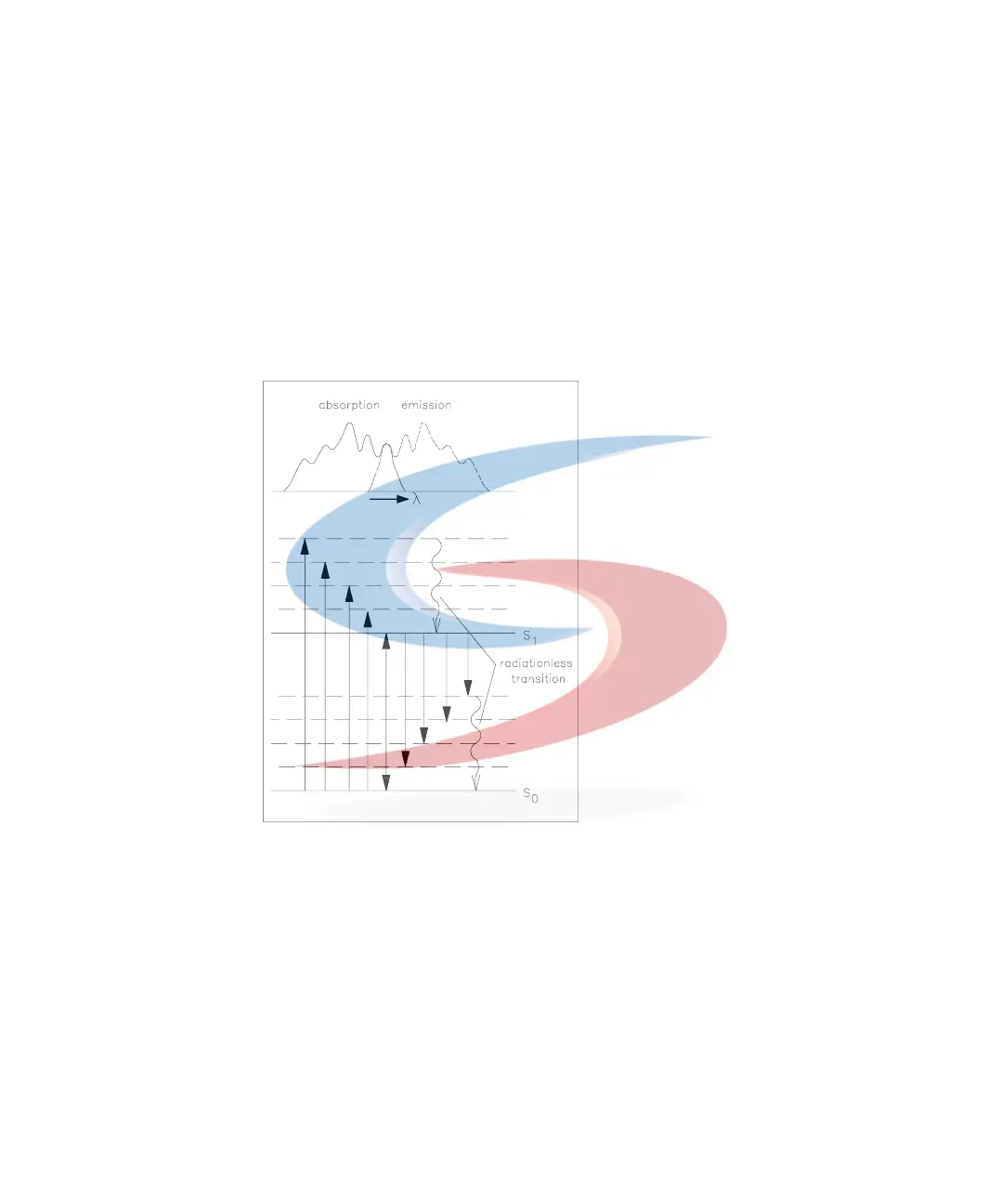
When a more complex molecule transforms from its ground energy state into
an excited state, the absorbed energy is distributed into various vibrational
and rotational sub-levels. When this, same molecule returns to the ground
state, this vibrational and rotational energy is first lost by relaxation without
any radiation. Then the molecule transforms from this energy level to one of
the vibrational and rotational sub-levels of its ground state, emitting light, see
Figure 74. The characteristic maxima of absorption for a substance is its
λ
EX
,
and for emission its
λ
EM
.
Photoluminescence is the collective name for two phenomena, fluorescence
and phosphorescence, which differ from each other in one characteristic
way--the delay of emission after excitation. If a molecule emits light 10
-9
to
10
-5
seconds after it was illuminated then the process was fluorescence. If a
molecule emits light longer than 10
-3
seconds after illumination then the
process was phosphorescence.
Figure 74 Relationship of Excitation and Emission Wavelengths
 Loading...
Loading...
















