Dexcom G5 Mobile System User Guide
339Technical Information
The Dexcom G5 Mobile System is an M-PED (Medical-Portable Electronic Device), which meets the
FAA RTCA /DO-160 edition G Section 20, Category T and Section section 21, Category M. It can be
used on aircraft according to the directions provided by the operator of the aircraft.
This device can withstand exposure to common electrostatic discharge (ESD) and electromagnetic
interference (EMI).
Guidance and Manufacturer’s Declaration –
Electromagnetic Immunity
The transmitter (P/N 9438-06) is intended for use in the electromagnetic environment specified
in the next table. The customer or the user of the transmitter should ensure that it is used in such
an environment.
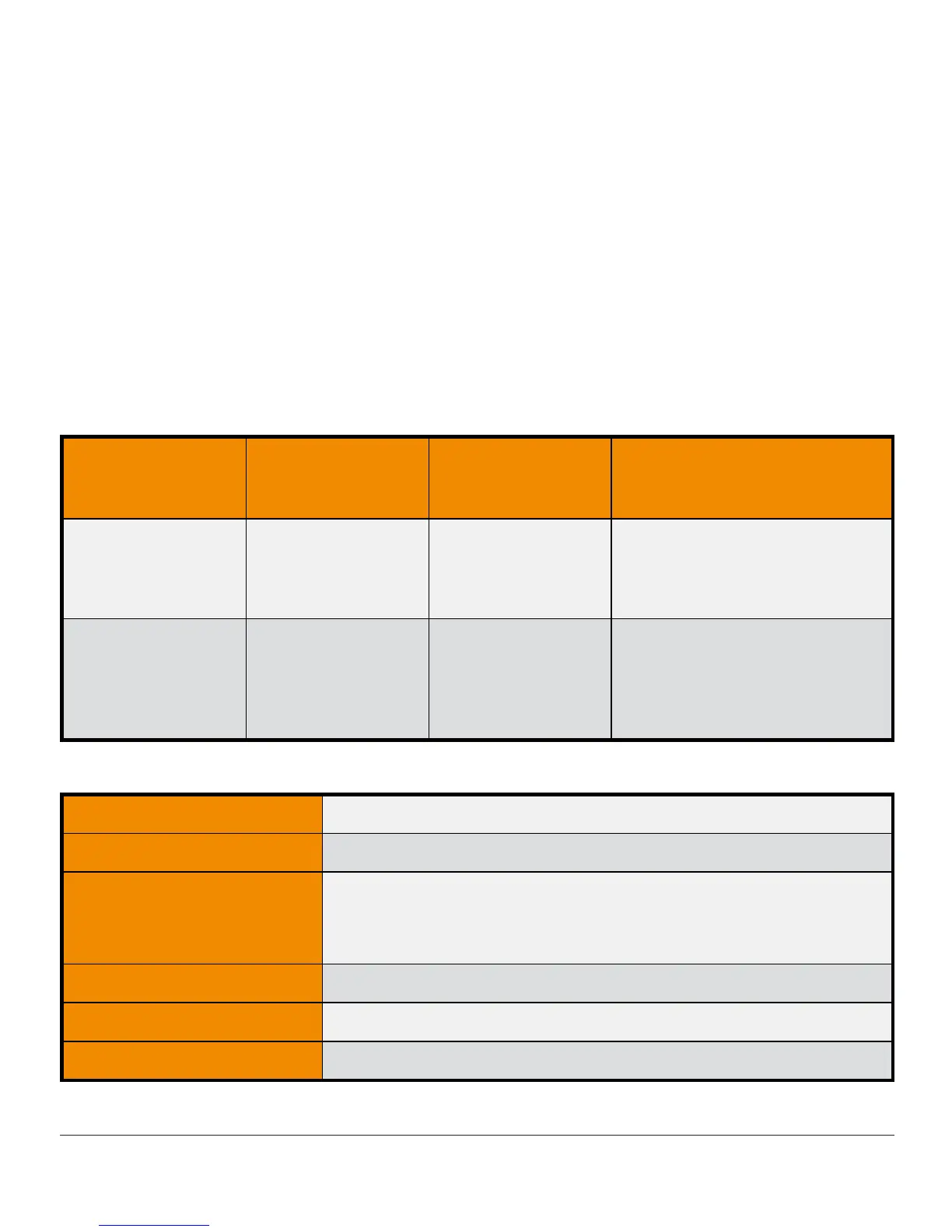
Transmitter Electromagnetic Immunity Specifications
Immunity Test
IEC 60601 Test
Level
Transmitter
Compliance
Level
Electromagnetic
Environment Guidance
Electrostatic
Discharge (ESD)
IEC 61000-4-2
± 8 kV Contact
± 15 kV Air
± 8 kV Contact
± 15 kV Air
Floors should be wood, concrete or
ceramic tile. If floors are covered
with synthetic material, the relative
humidity should be at least 30%.
Power Frequency
(50/60 Hz) Magnetic
Field
IEC 61000-4-8
30 A/m 30 A/m
Magnetic fields from common
appliances are not expected to
affect the system.
Receiver Product Specifications
Part Number
MT22949
Reading Frequency
Every 5 minutes
Dimensions
Length: 4.2 inches
Width: 2.5 inches
Thickness: 0.55 inches
TX/RX Frequencies
2.402-2.480 GHz
Bandwidth
1.22 MHz
Maximum Output Power
2.5 mW EIRP
(Continued on next page)

 Loading...
Loading...