HemoSphere Advanced Monitor 12 Advanced Features
160
SVV, dP/dt and Ea
dyn
are intended as integrative decision support parameters to guide an interventional
treatment of SV or SV and MAP.
12.1.10 Clinical Validation
A clinical validation study was undertaken to assess the measurement performance of the P(↓BP) parameter.
This study included 350 patients consisting of 52 surgical patients (OR) and 298 intensive care unit patients
(ICU). Table 12-5 provides the patient demographics.
The 52 OR patients can be further stratified in two groups those who underwent high risk non-cardiac
surgery (n=25, 48.1%) and those who underwent liver surgery (n=27, 51.9%).
The 298 ICU patients were admitted for various reasons, the most prevalent of which were post-surgery
(n=141, 47.3%), sepsis (n=65, 21.8%), and septic shock (n=25, 8.4%).
Table 12-8 provides the further details.
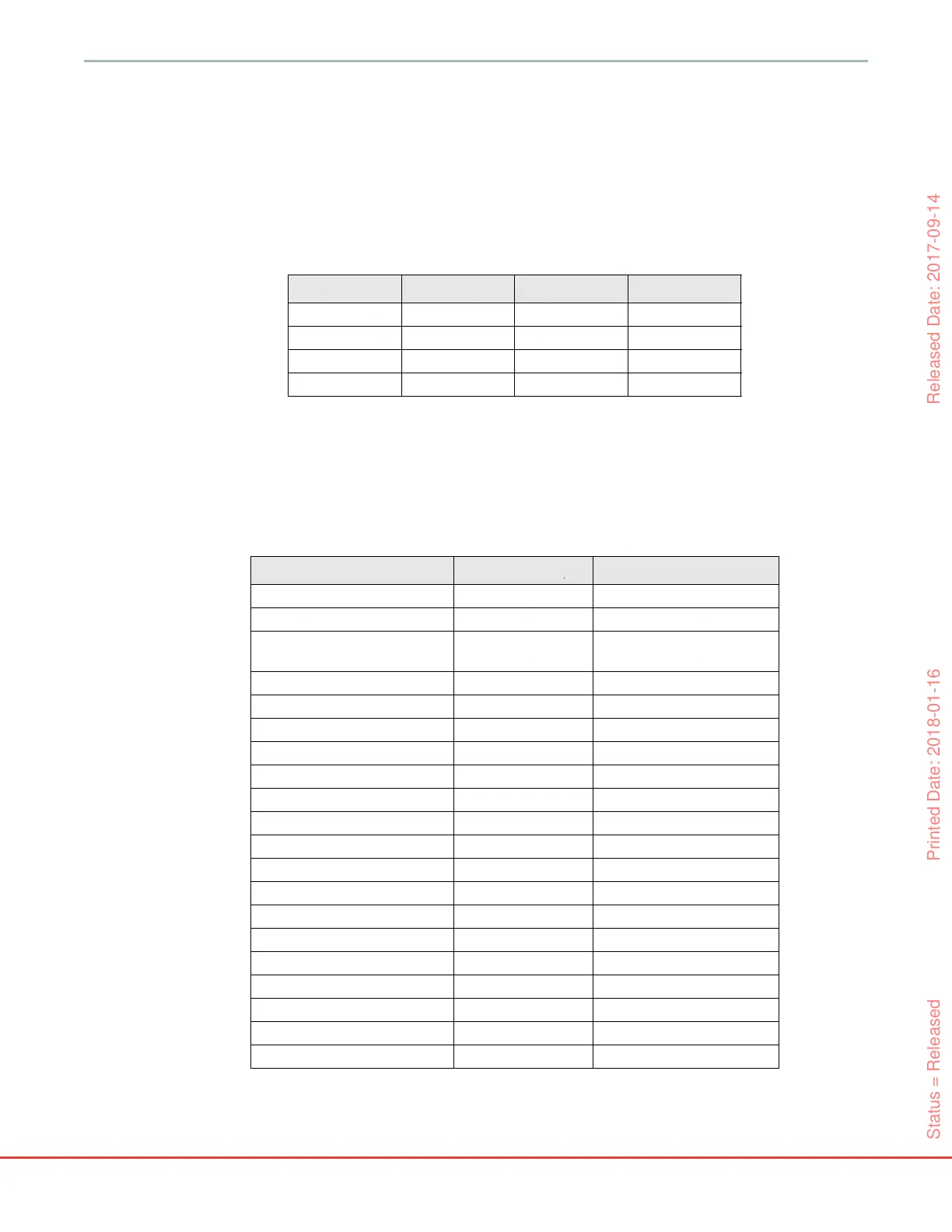
Table 12-5 Patient Demographics
Type Validation OR ICU
# of Patients
350 52 298
Gender (Male)
220 29 191
Age
61.9±14.7 58.3±11.3 62.6±15.1
BSA 1.9±0.2 1.8±0.2 1.9±0.3
Table 12-6 ICU Admission Details
ICU # of patients % of total 298
post cardiac surgery 70 23.5
sepsis 65 21.8
post-surgery
(non-cardiac/liver)
46 15.4
post-liver surgery 25 8.4
septic shock 25 8.4
cardiac 12 4.0
infarction 8 2.7
respiratory/pulmonary 8 2.7
severe hypovolemia 8 2.7
cardiogenic shock 7 2.3
other 5 1.7
hemorrhage 4 1.3
unknown 4 1.3
aneurysm 2 0.7
poison 2 0.7
renal failure 2 0.7
stroke 2 0.7
diabetes 1 0.3
infectious disease 1 0.3
liver 1 0.3
Status = Released Printed Date: 2018-01-16 Released Date: 2017-09-14
 Loading...
Loading...