220
HemoSphere Advanced Monitor G Guidance and Manufacturer’s Declaration
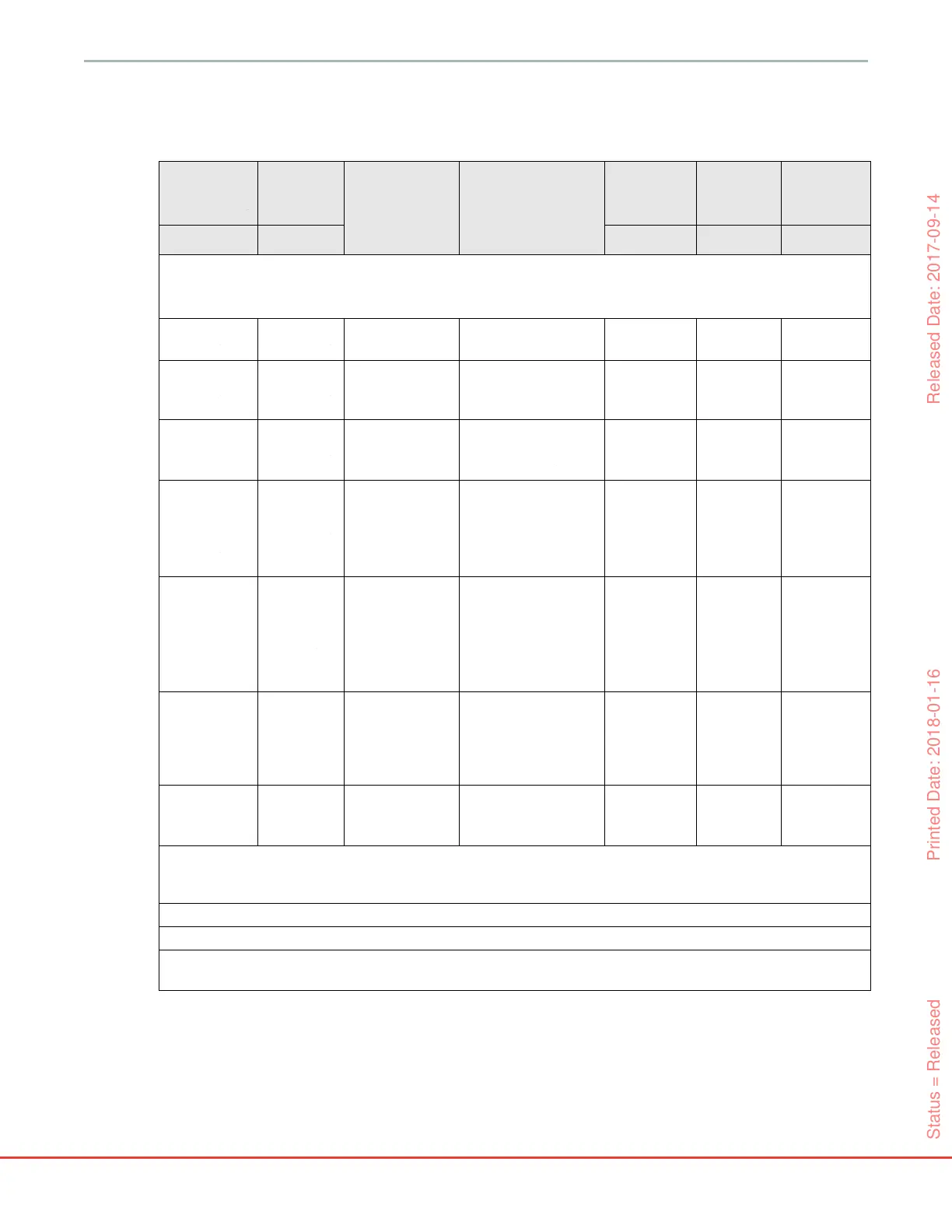
Table G-2 Guidance and Manufacturer's Declaration - Immunity to RF wireless communications
equipment
Test
Frequency
Band
1
Service
1
Modulation
2
Maximum
Power
Distance
Immunity
Test
Level
MHz MHz W Meters (V/m)
The HemoSphere advanced monitor is intended for use in the electromagnetic environment
specified below. The customer or user of the HemoSphere advanced monitor should ensure
that it is used in such an environment.
385 380 - 390 TETRA 400
Pulse modulation ²
18 Hz
1.8 0.3 27
450
430 - 470
GMRS 460,
FRS 460
FM ³
± 5 kHz deviation 1
kHz sine
20.328
710
745
780
704 - 787
LTE Band 13,
17
Pulse modulation ²
217 Hz
0.2 0.3 9
810
870
930
800 - 960
GSM 800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse modulation ²
18 Hz
20.328
1720
1845
1970
1700 -
1900
GSM 1800;
CDMA 1900;
GSM 1900;
DECT;
LTE Band 1, 3,
4, 25; UMTS
Pulse modulation ²
217 Hz
20.328
2450
2400 -
2570
Bluetooth,
WLAN,
802.11 b/g/n,
RFID 2450,
LTE Band 7
Pulse modulation ²
217 Hz
20.328
5240
5500
5785
5100 -
5800
WLAN
802.11a/n
Pulse modulation ²
217 Hz
0.2 0.3 9
NOTE If necessary to achieve the IMMUNITY TEST LEVEL, the distance between the transmitting antenna and
the ME EQUIPMENT or ME SYSTEM may be reduced to 1 m. The 1 m test distance is permitted by IEC 61000-
4-3.
¹ For some services, only the uplink frequencies are included.
² The carrier shall be modulated using a 50 % duty cycle square wave signal.
³ As an alternative to FM modulation, 50 % pulse modulation at 18 Hz may be used because while it does not
represent actual modulation, it would be worst case.
Status = Released Printed Date: 2018-01-16 Released Date: 2017-09-14
 Loading...
Loading...