To help ensure patient safety when using an endocavity transducer, observe the
following guidelines:
• Scrutinize the entire transducer before each use (see the "Transducer Care"
section).
• Operate the transducer properly.
• Do not allow water or other liquids to drip onto the transducer connector,
the interior of the system, or the keyboard.
• Use sterile ultrasound transmission gel when performing all endocavity
studies.
• Protective covers are recommended for endocavity procedures; the
protective covers are mandatory in China and Japan.
WARNINGS
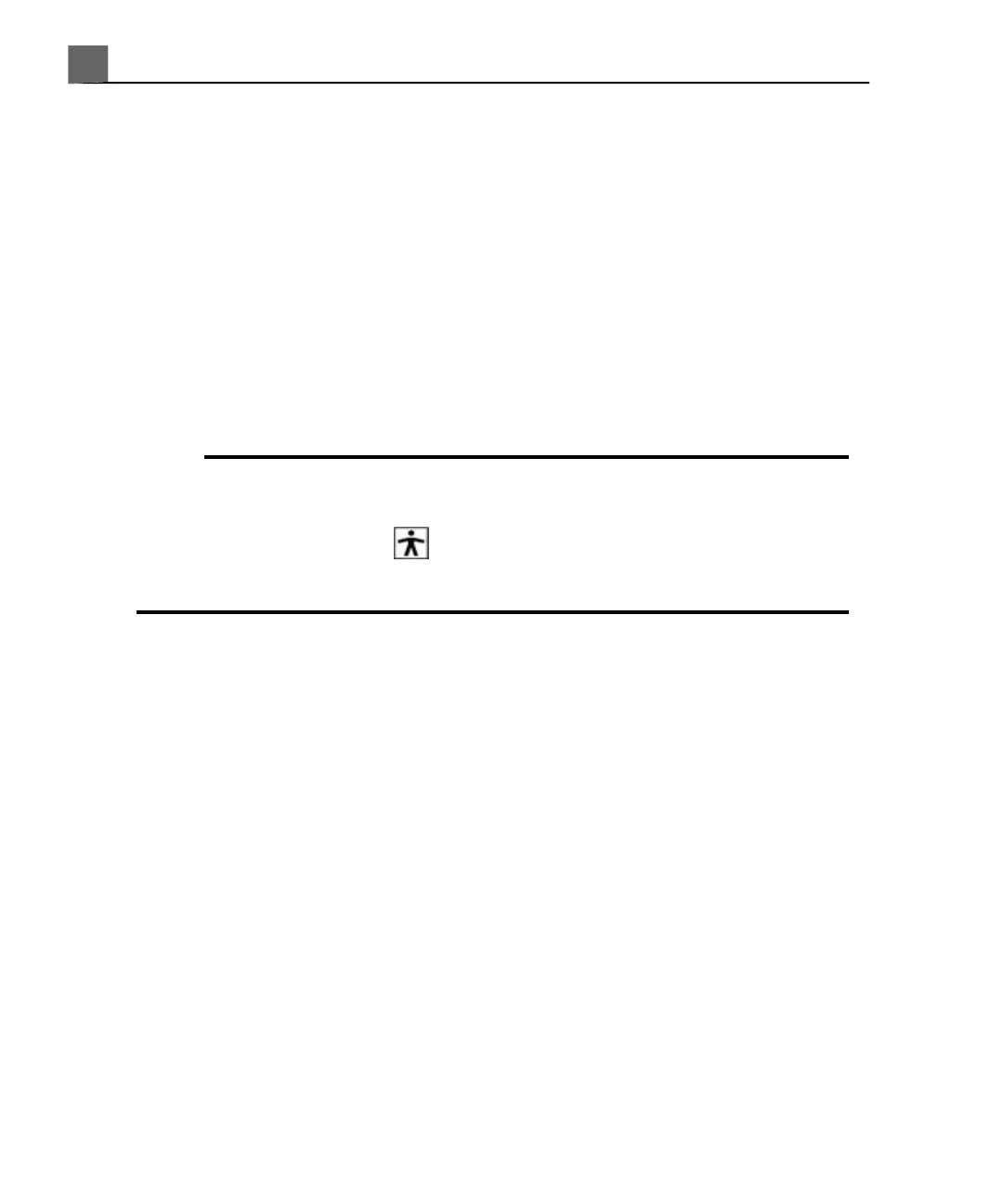
• All endocavity studies must be performed with a Type BF classified
transducer. If your transducer is not labeled on the transducer connector
with a Type BF symbol, , contact your Philips service representative.
• Always remove the transducer from the patient before defibrillation.
Preparing Transducers for Endocavity Use
1. Place 20 cc of sterile gel or saline into the transducer cover.
2. Carefully inspect each transducer cover before use, and discard it if you find
tears or blemishes. Also inspect each transducer cover after use. If you find
a tear, the patient or the transducer may have been contaminated.
3. Insert the transducer into the transducer cover and unfurl the transducer
cover until it covers the transducer and its cable. The cover must be unfurled
far enough to maintain the sterile field.
4. Use a sterile elastic band or clip to hold the proximal end of the transducer
cover in place.
5. Ensure that wrinkles and bubbles over the face of the transducer are
minimized. Check the transducer cover for tears or damage before proceeding.
6. When operating the transducer, make sure that proper orientation is
maintained to avoid interpretation confusion.
iU22 User Manual
266
4535 614 45861
Endocavity Transducers
11
 Loading...
Loading...











