probe in a storage cabinet with filtered air flow and/or by using a disposable storage
cover placed over the probe
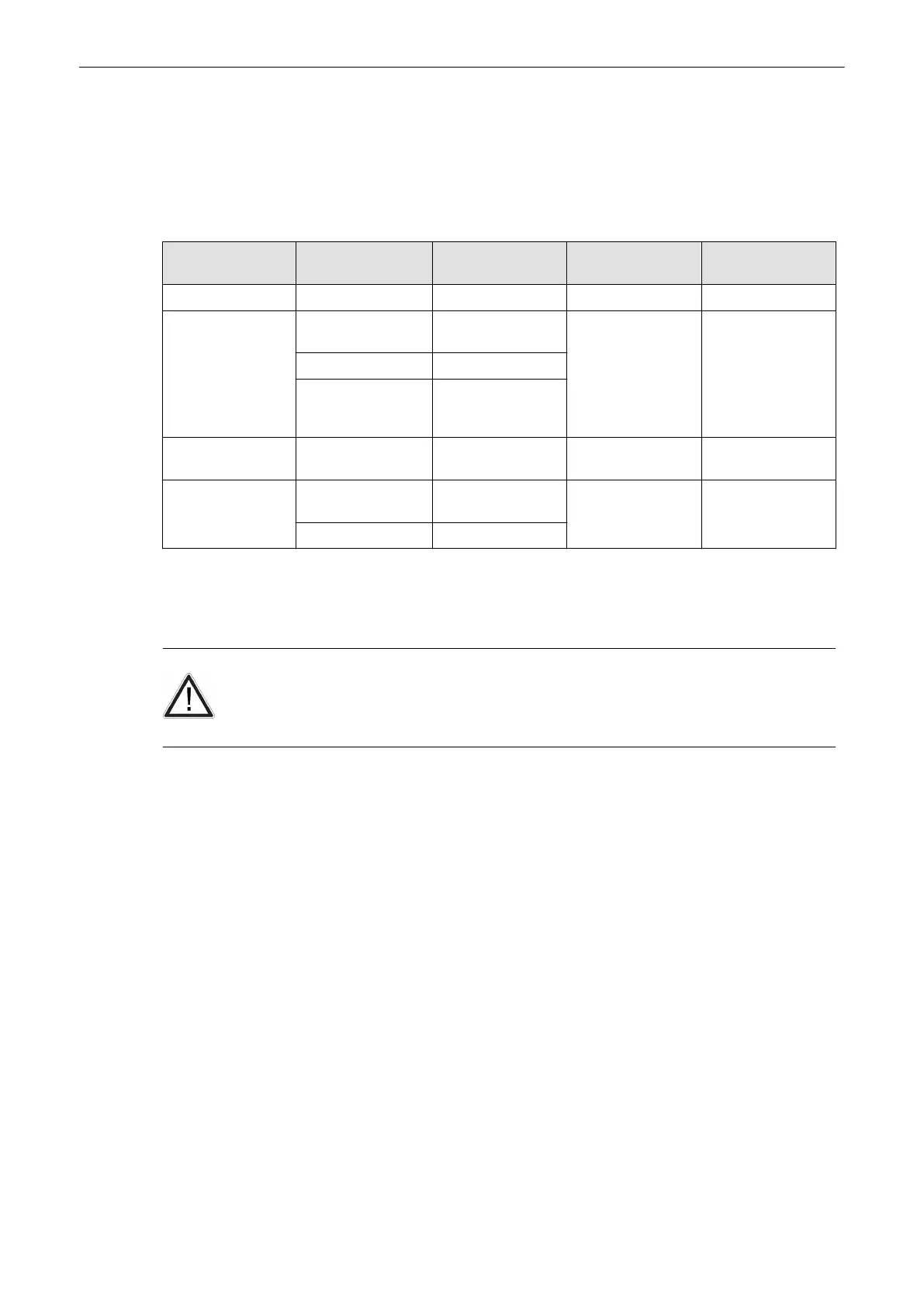
Chemicals Used for Efficacy Validation
The table below lists the products and intended use (clean, Intermediate-Level Disinfection,
High-Level Disinfection) that were validated.
Product Type Trade Name Manufacturer Minimum Contact
Time
Active Ingredient
Cleaning (Wipe) Oxivir® Tb Diversey N/A Hydrogen Peroxide
Enzymatic Detergent
(Soak)
Enzol® (Cidezyme®) Advanced Sterilization
Products® (J&J)
1-Minute Soak Proteolytic Enzymes
MetriZyme™ Metrex™
Prolystica® 2X
Concentrate Presoak
& Cleaner
Steris
Intermediate-level
Disinfectant (wipe)
Oxivir® Tb Diversey 10-Minute Exposure Hydrogen Peroxide
High-level
Disinfectant (Soak)
Cidex® OPA Advanced Sterilization
Products® (J&J)
10-Minute Soak Ortho-phthalaldehyde
McKessen OPA/28 McKesson
Table 5-3 Chemicals used for Efficacy Validation
A full list of chemicals tested for compatibility is available at the GE Probe Web Site.
Covering the Transducer using a Sterile, Protective Sheath
Caution
Protective barriers may be required to minimize disease transmission. Probe sheaths are
available for use with all clinical situations where infection is a concern. Use of legally
marketed, sterile probe sheaths is recommended* for intra-cavitary and intra-operative
procedures.
1. Place an appropriate amount of gel inside the protective sheath and/or on the transducer
face.
Note
Failure to use imaging gel may result in poor image quality.
2. Insert transducer into sheath, making sure to use proper sterile technique*. Pull cover
tightly over transducer face to remove wrinkles and air bubbles, taking care to avoid
puncturing the sheath.
* The use of a sterile sheath is recommended for every semi-critical use of the probe.
Endoscopic, rectal, and transvaginal probes should be used with a single-use sterile
sheath. (Market Clearance of Diagnostic Ultrasound Systems and Transducers, FDA
June 27, 2019)
Probes and Biopsies
Voluson™ SWIFT / Voluson SWIFT+ Instructions For Use
5831612-100 R
evision 4 5-15
 Loading...
Loading...