5. Sensors and optical system ABL90 FLEX reference manual
5-6
The calibration equation
The calibration equation expresses the relationship between the electrical
measurement at a sensor and the concentration of the substrate specific to the
sensor.
The calibration line forms the basis of the scale used by the analyzer to convert
electrical measurements to concentrations.
Each sensor has a unique calibration equation.
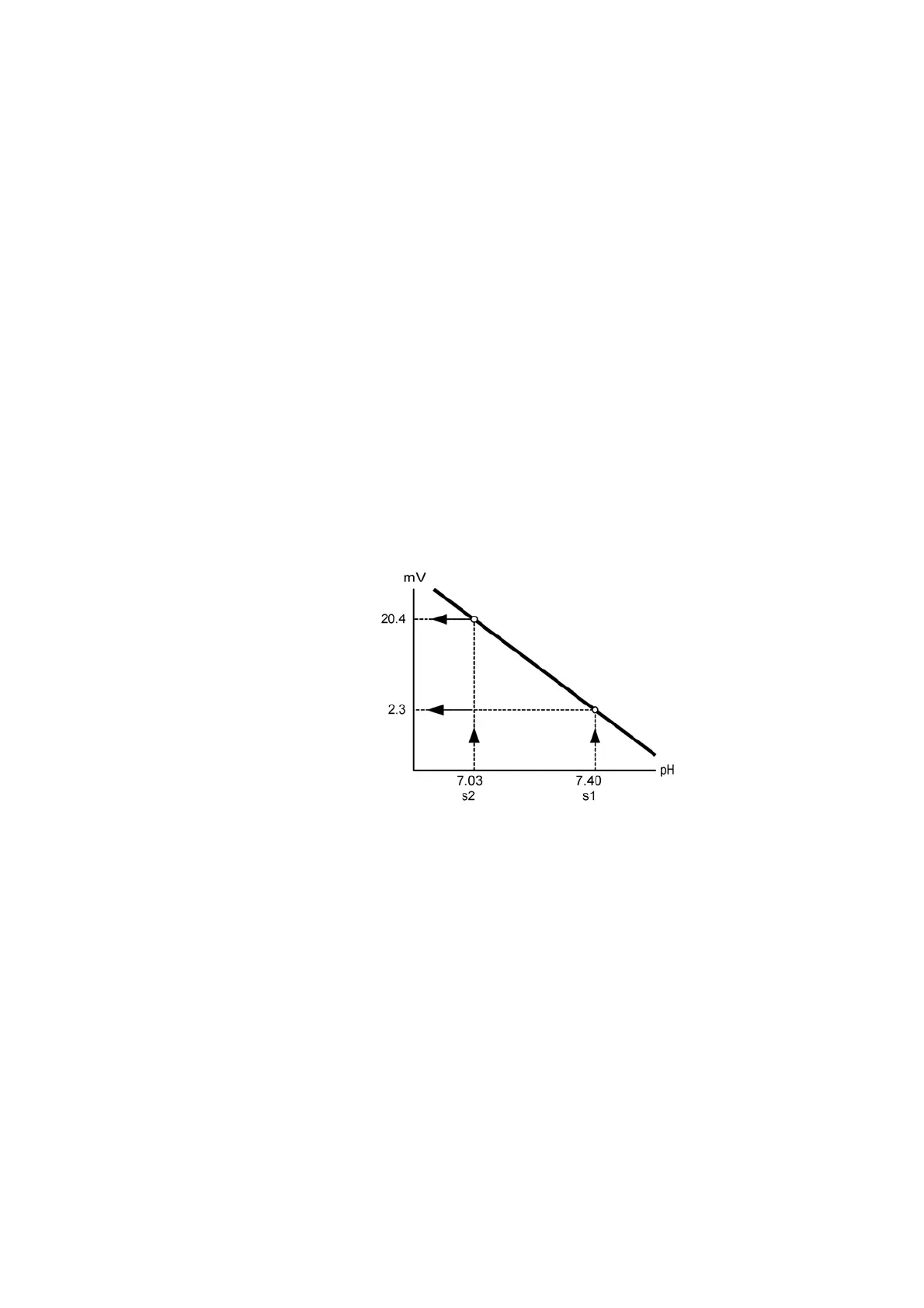
In the following example of a potentiometric sensor, the pH sensor is used to
illustrate how this equation is derived from two solutions of known pH. The pH
value as graphed is a linear scale. All other electrolyte values, if graphed, would
be expressed as log
10
(a
ion
).
Solution 1 (s1) has a pH of 7.40, which gives a potential reading of 2.3 mV.
Solution 2 (s2) has a pH of 7.03, which gives a potential reading of 20.4 mV.
These two values are plotted on a graph.
The relationship between potential and pH is linear so a line can be drawn
between the two points, as shown in the diagram below:
The calibration line now forms the scale used to convert the potential measured
at the pH sensor during sample analysis to an actual pH value.
Definition
Use
Deriving the
calibration
line
Scale

 Loading...
Loading...