5. Sensors and optical system ABL90 FLEX reference manual
5-18
Measuring principle of the pH and electrolyte sensors
The pH and electrolyte sensors are measured according to the potentiometric
measuring principle, where the potential of an electrode chain recorded at a
voltmeter is related to the concentration of a substance via the Nernst equation.
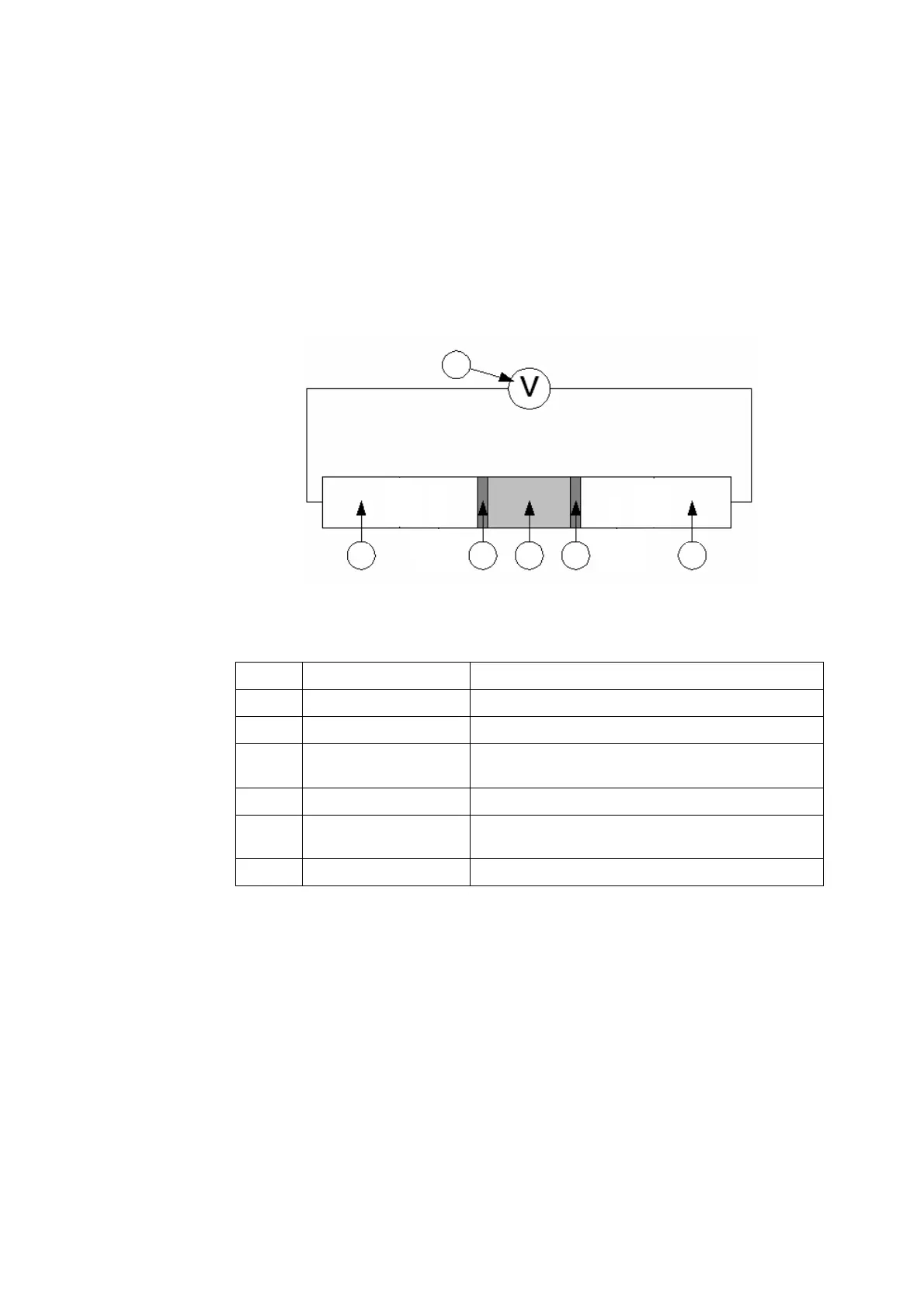
The electrode chain (or electrical circuit) set up to measure pH/electrolytes is
illustrated in the following diagram:
1
2
3
4 5
6
The electrode chain describes an electrical circuit consisting of the following:
Item Part Function
1 Voltmeter Measures the voltage potential in the circuit.
2 Reference electrode Provides electrical connection to the voltmeter.
3 Liquid junction Point of contact between the reference sensor
and the sample.
4 Sample The unknown liquid being measured.
5 Membrane An ion-sensitive membrane, which is sensitive
to H
+
/electrolyte ions.
6 Solid-state contact Provides electrical connection to the voltmeter.
Every element in the electrode chain contributes a voltage to the total potential
drop through the chain. Thus:
When immersed in the appropriate electrolyte solution, both electrodes exhibit
separate potentials
The membrane junctions between the sample and electrolyte solutions also
exhibit separate potentials
The total potential across the electrode chain, therefore, is the sum of these
separate potentials, all but one of which are known and constant, as outlined in
the table on next page.
Potentiometric
measuring
principle
Electrode chain
Parts and
description
Electrode chain
potential

 Loading...
Loading...