7. Performance characteristics ABL90 FLEX reference manual
7-18
Performance test results – bilirubin
The reference method for total bilirubin is a spectrofotometric method (wet chemistry
based on a method from Bayer Healthcare, Tarrytown USA.
The method is calibrated using NIST SRM916a Bilirubin.
The method is performed by the Laboratory Unilabs AS., Denmark.
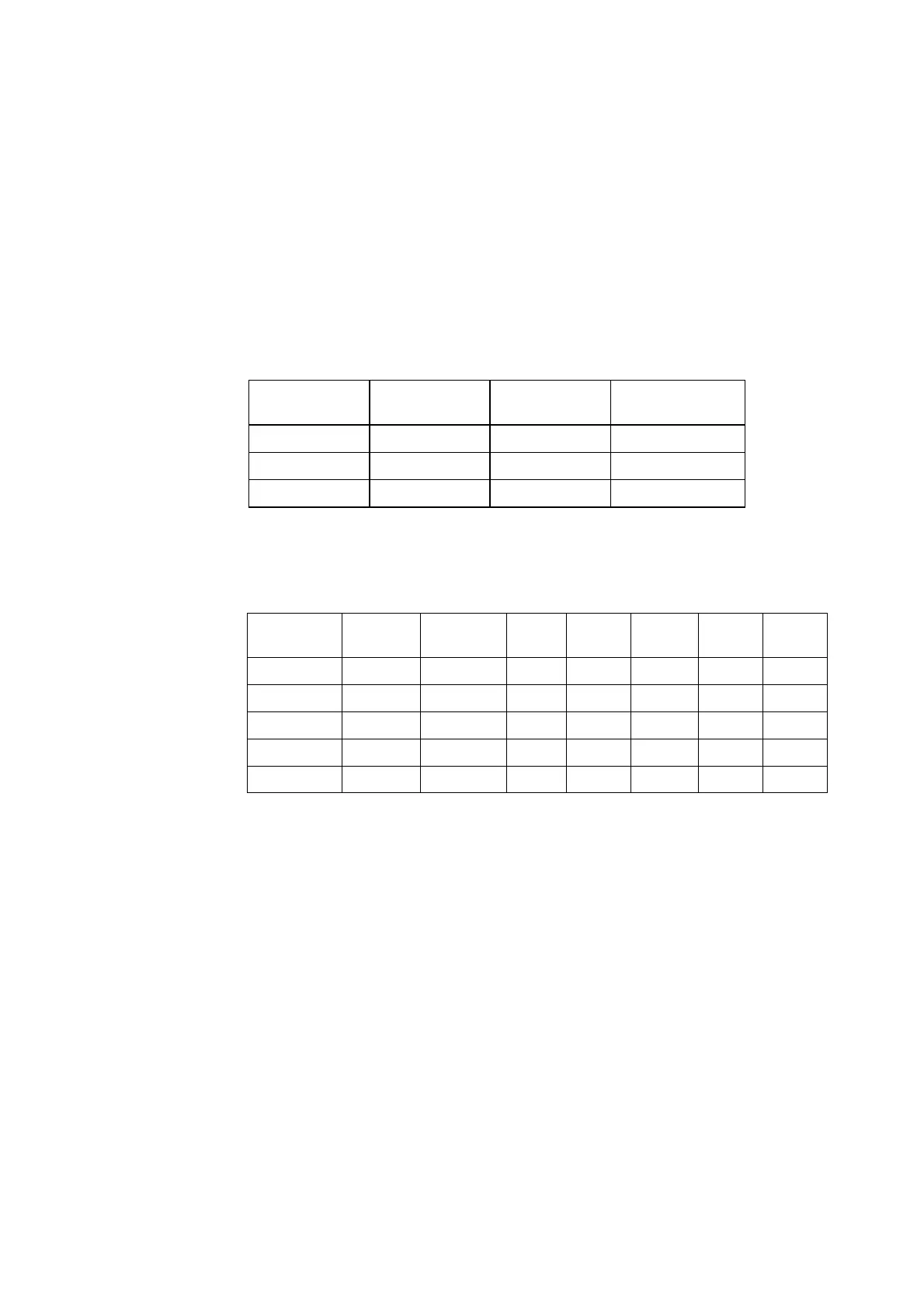
Setup
:
HbF correction is not activated
ctBil
(µmol/L)
ctHb (g/dL)
Bias
Prim.ref
N
0 15 0.8 3
200 15 4.7 3
400 15 4.7 3
Bias
ABL90-Prim.ref
= Bias
ABL90-ABL735
+ Bias
ABL735-Prim.ref
ctBil
(µmol/L)
ctHb
(g/dL)
Bias
Sec.ref
S
0
S
X
CV
X
% TE
A
TE
A
(%)
8 15 1.0 2.7 7.1 89 14.9 186
100 15 0.2 3.2 9.7 9.7 19.2 19.2
200 15 -4.8 3.6 12.7 6.4 29.7 14.9
400 15 -5.3 4.8 13.9 3.5 32.5 8.1
600 15 -11.7 5.9 18.0 3.0 47.0 7.8
NOTES:
a. Adult/fetal blood, pH = 7.4 0.1, normal MCHC and albumin variation. Spiked
with unconjugated bilirubin.
The purpose of the bilirubin external tests was to make a regression study of ABL90
bilirubin against reference hospital analyzers on hospital neonatal blood samples.
A limited study was performed on hospital adult samples [Ref. 13].
For neonatal use: The bilirubin method has been evaluated on whole blood.
The allowed analytical error is
10% to satisfy average
clinical requirements for bilirubin measurement
[16,17,18,19,20]. For whole blood the analytical error is
slightly higher.
Reference
method
Bias
Prim.ref
Bias
Sec.ref
External test
results

 Loading...
Loading...