Qualification Schedule | 217
Syringe Dispenser Qualification Tests:
l Perform the Syringe Dispense Precision & Accuracy Test on page 236
Qualification Schedule
The following schedule defines the factory-recommended intervals for verification
tests for an instrument used two to five days a week.The schedule assumes that
the MultiFlo FX is properly maintained as outlined in the Recommended Maintenance
Schedule on page 176.
n Note: An instrument qualification package (PN 1260521) is available for
purchase. The package contains thorough procedures for performing
Installation Qualification, Operational Qualification and Performance
Qualification (IQ-OQ-PQ) and preventative maintenance (PM). Extensive
Checklists and Logbooks are included for recording results. Contact your local
dealer for more information.
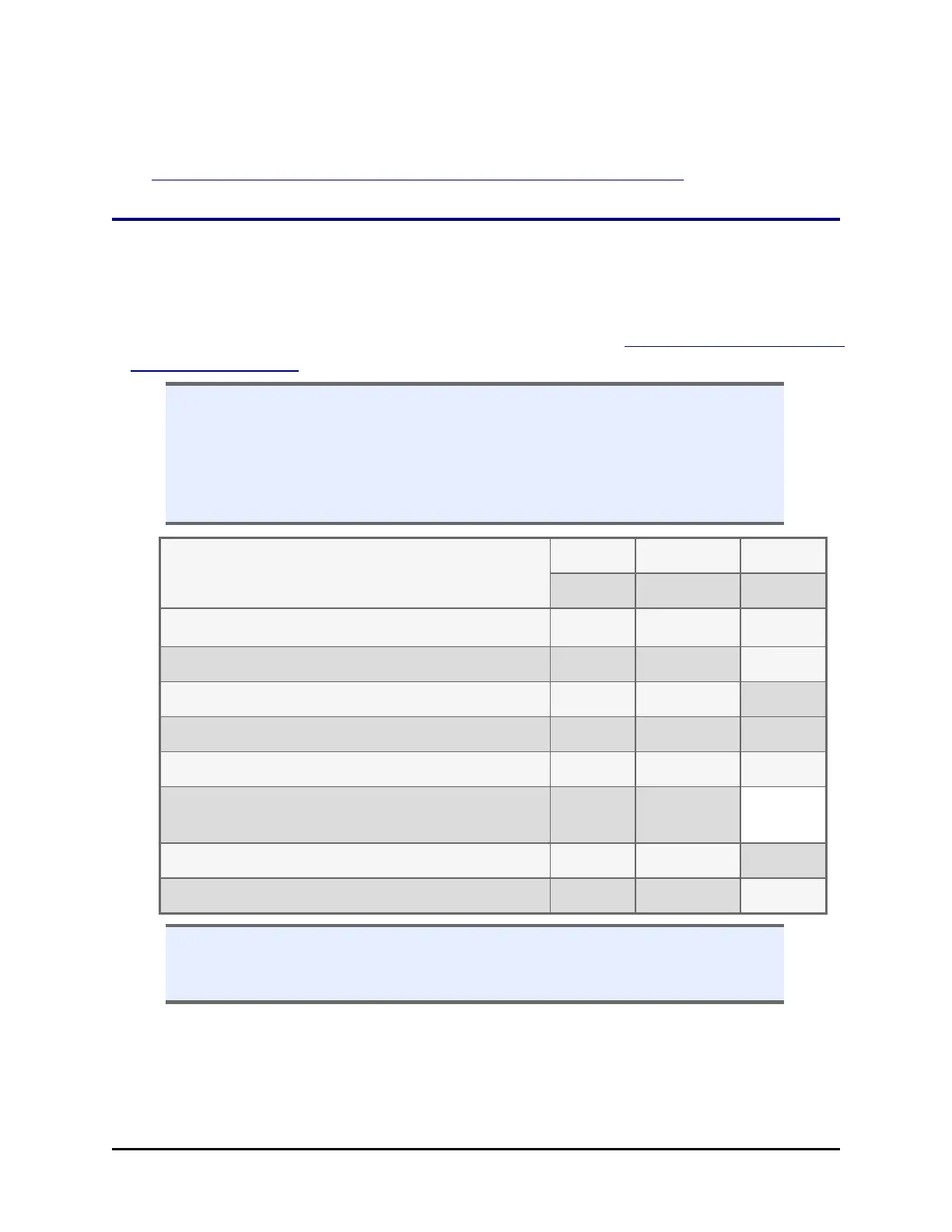
Tests
IQ OQ PQ
Initially Annually Monthly
Unpacking, Installation, and Setup
ü
System Self Test and Checksum Test
ü ü ü
Shake Test
ü
Washer Evacuation Efficiency Test
ü ü
Washer Dispense Precision & Accuracy Test
ü ü
Peri-pump Dispense Precision and Accuracy
Test
ü ü
Syringe Dispense Precision and Accuracy Test
ü ü
Run Assay
ü
n Important! The risk factors associated with your assays may require that the
Operational and Performance Qualification procedures be performed more or
less frequently than shown above.
BioTek Instruments, Inc.
 Loading...
Loading...