i-2 Vivid S70 / S60 – User Manual
BC092760-1EN
01
Regulatory Requirements
Conformance Standards
The GE Healthcare product families are tested to meet all
applicable requirements and relevant standards per the
countries in which the product will be sold. Any changes to
accessories, peripheral units, or any other part of the system
must be approved by the manufacturer: GE Vingmed Ultrasound
AS. Ignoring this advice may compromise the regulatory
approvals obtained for the product.
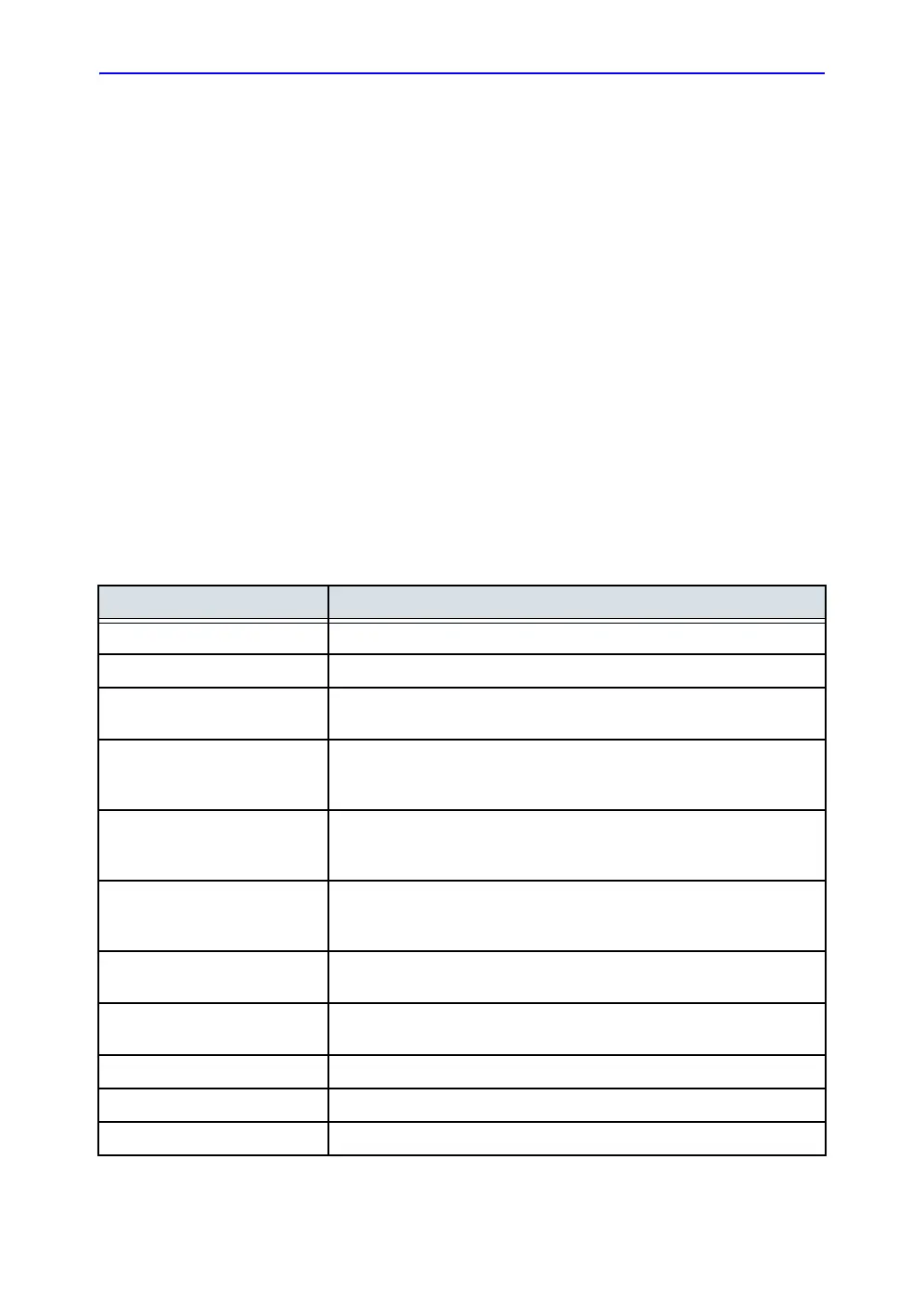
This product complies with the regulatory requirements of the
following:
Standard/Directive Scope
93/42/EEC Medical Devices Directive (MDD)
2012/19/EU Waste Electrical and Electronic Equipment (WEEE)
2011/65/EU Directive on the restriction of the use of certain hazardous substances in
electrical and electronic equipment (ROHS)
IEC/EN 60601-1
ANSI/AAMI ES60601-1
CAN/CSA-C22.2 No. 60601-1
Medical Electrical Equipment - Part 1. General requirements for basic
Safety and essential performance
IEC/EN 60601-2-37 Medical electrical equipment - Part 2-37. Particular requirements for the
basic safety and essential performance of ultrasonic medical diagnostic
and monitoring equipment
IEC/EN 60601-1-2 Medical Electrical Equipment - Part 1-2. General requirements for basic
safety and essential performance - Collateral standard: Electromagnetic
disturbances - Requirements and tests
IEC/EN 60601-1-6 Medical Electrical Equipment - Part 1-6. General requirements for basic
safety and essential performance - Collateral standard: Usability
NEMA/AIUM UD-3 Standard for real-time display of thermal and mechanical acoustic output
indices on diagnostic ultrasound equipment.
IEC/EN 62304 Medical Device Software - Software life-cycle processes
IEC/EN 62366 Medical Devices - Application of usability engineering to medical devices
ISO 10993-1 Biological evaluation of medical devices
 Loading...
Loading...