Safety
2-28 Vivid S70 / S60 – User Manual
BC092760-1EN
01
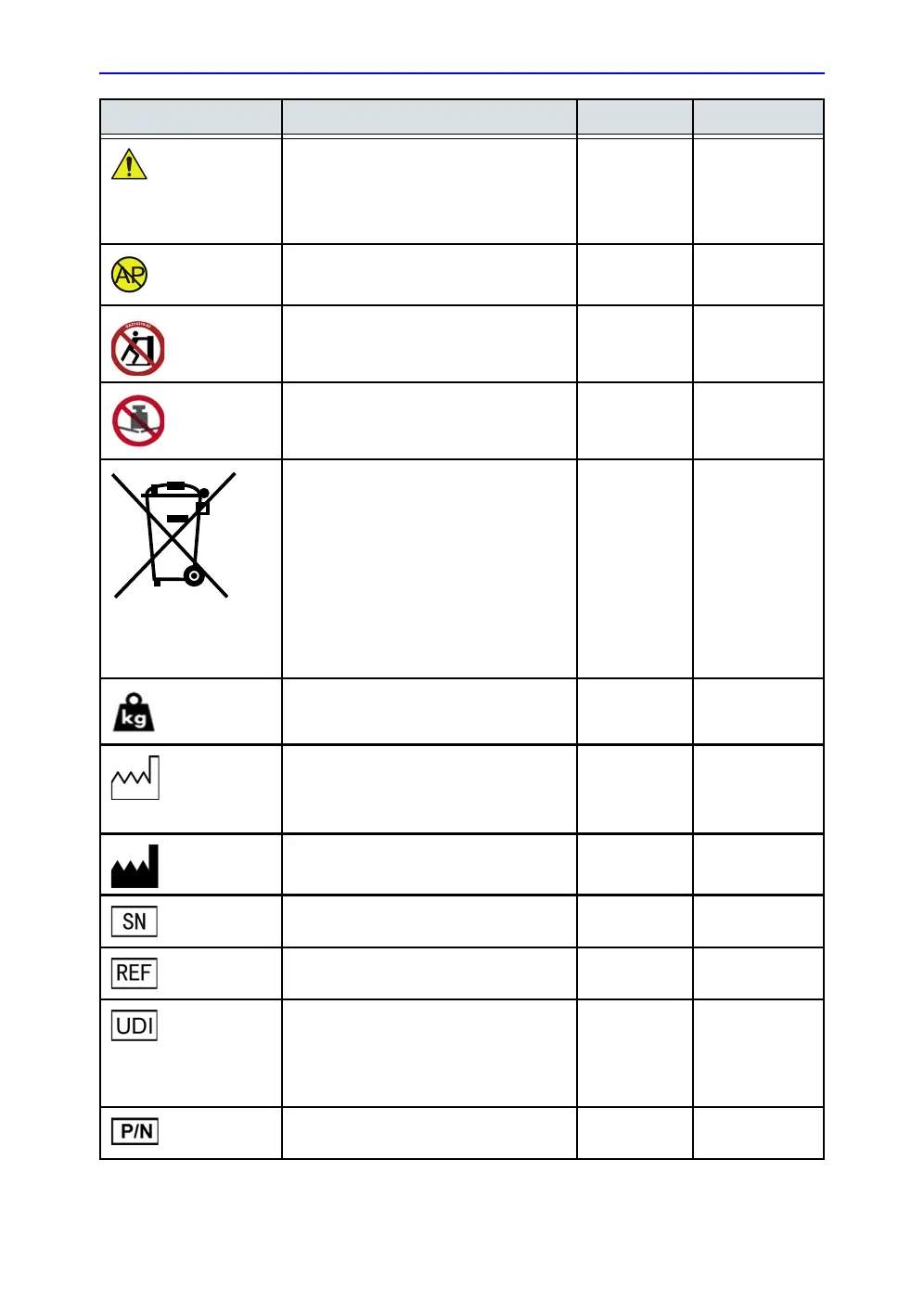
Attention - Consult accompanying
documents: Alerts the user to refer to the
user documentation when complete
information cannot be provided on the
label.
Various ISO 7010-W001
The system is not designed for use with
flammable anesthetic gases.
Rear of
system
N/A- by GE
Healthcare
CAUTION - Do not push the system
sideways when casters are in break
position. Instability may occur.
Top console
(both sides)
ISO 7010-P017
DO NOT place objects on the surface of
the rear of the main display while folded.
Display rear
panel
ISO 7010-P012
This symbol indicates that the waste of
electrical and electronic equipment must
not be disposed as unsorted municipal
waste and must be collected separately.
Please contact the manufacturer or other
authorized disposal company to
decommission your equipment.
The disassembly and parts disposition
procedure is located on the card cage
front cover. To access to the procedure,
remove the right side panel.
Rear of
system
EN 50419
System weight Rear of
system
N/A- by GE
Healthcare
Date of manufacture: The date could be a
year, year and month, or year, month and
day, as appropriate. See ISO 8601 for
date formats.
Rear of
system
ISO 7000-2497
Manufacturer name and address Rear of
system
ISO 7000-3082
Serial number Rear of
system
ISO 7000-2498
Brand and model identifier Rear of
system
ISO 7000-2493
Unique Device Identification (UDI). Every
system has a unique marking for
identification. Scan or enter the UDI
information into the patient health record
as required by governing laws.
Rear of
system
N/A- by GE
Healthcare
Device part number identifier Rear of
system
N/A- by GE
Healthcare
Label Purpose Location
Standard
 Loading...
Loading...