Regulatory and Safety Information
Category Classification
Degree of safety of application in the presence
of a flammable anesthetic mixture with air or
with oxygen or with nitrous oxide
Equipment is not suitable for use in the presence
of a flammable anesthetic mixture with air or
with oxygen or with nitrous oxide
Method(s) of sterilization or disinfection
recommended by the manufacturer
Not applicable
Mode of operation Continuous operation
Certification Information
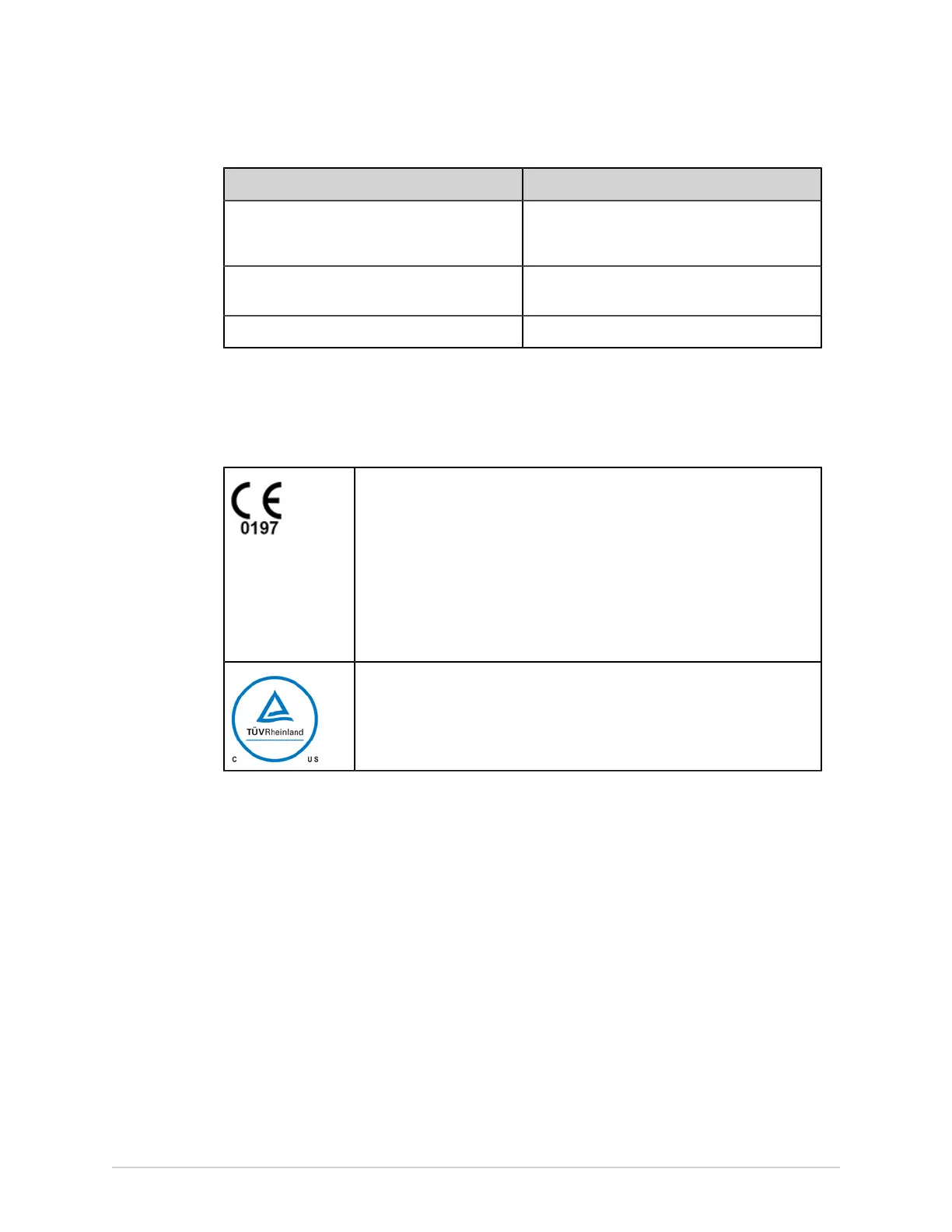
Table 130: Certification Information
This system bears CE mark 0197 indicating it conforms with the provisions of
Council Regulation EU 2017/745 concerning medical devices, and it fulfills the
requirements of Annex I of this regulation.
The system is in radio-interference protection class B in accordance with EN
55011. The country of manufacture is indicated on the equipment labeling.
The product complies with the requirements of standard EN 60601-1-2
“Electromagnetic Compatibility – Medical Electrical Equipment”.
The medical device has a lifetime of 7 years with respect to the Council
Regulation EU 2017/745 Annex I, Requirement 6.
Medical Equipment
With respect to electric shock, fire, and mechanical hazards only in
accordance with IEC 60601-1, and CAN/CSA C22.2 NO. 601.1.
Recording ECGs during Defibrillation
This equipment is protected against the effects of cardiac defibrillator discharge
to ensure recovery, as required by test standards. The patient signal input of the
acquisition module is defibrillation-proof. It is not necessary to remove the ECG
electrodes prior to defibrillation.
When using stainless steel or silver electrodes, a defibrillator discharge current may
cause the electrodes to retain a residual charge causing a polarization or DC offset
voltage. This electrode polarization blocks acquisition of the ECG signal. To avoid this
condition, if there is a situation where a defibrillation procedure is necessary, use
non-polarizing electrodes such as silver/silver-chloride types, which do not form a DC
offset voltage when subjected to a DC current.
If you use polarizing electrodes, GE Healthcare recommends disconnecting the
leadwires from the patient before delivering the shock.
5864335-001-1 MAC
™
5 A4/MAC
™
5 A5/MAC
™
5 Lite 345
 Loading...
Loading...