ECG Accessories Accessories
18 - 2 TM80 Telemetry Monitor Operator’s Manual
The accessories listed in this chapter comply with the requirements of IEC 60601-1-2
when in use with the device. The accessory material that contacts the patients has
undertaken the bio-compatibility test and is verified to be in compliance with ISO
10993-1. For details about the accessories, refer to the instructions for use provided with
the accessory.
18.1 ECG Accessories
18.1.1 ECG Electrodes
WARNING
• Use accessories specified in this chapter. Using other accessories may
cause damage to the monitor or not meet the claimed specifications.
• Single-use accessories are not designed to be reused. Reuse may cause a
risk of contamination and affect the measurement accuracy.
• Check the accessories and their packages for any sign of damage. Do not
use them if any damage is detected.
• Use the accessories before the expiry date if indicated.
• The disposable accessories shall be disposed of according to the hospi-
tal's regulations.
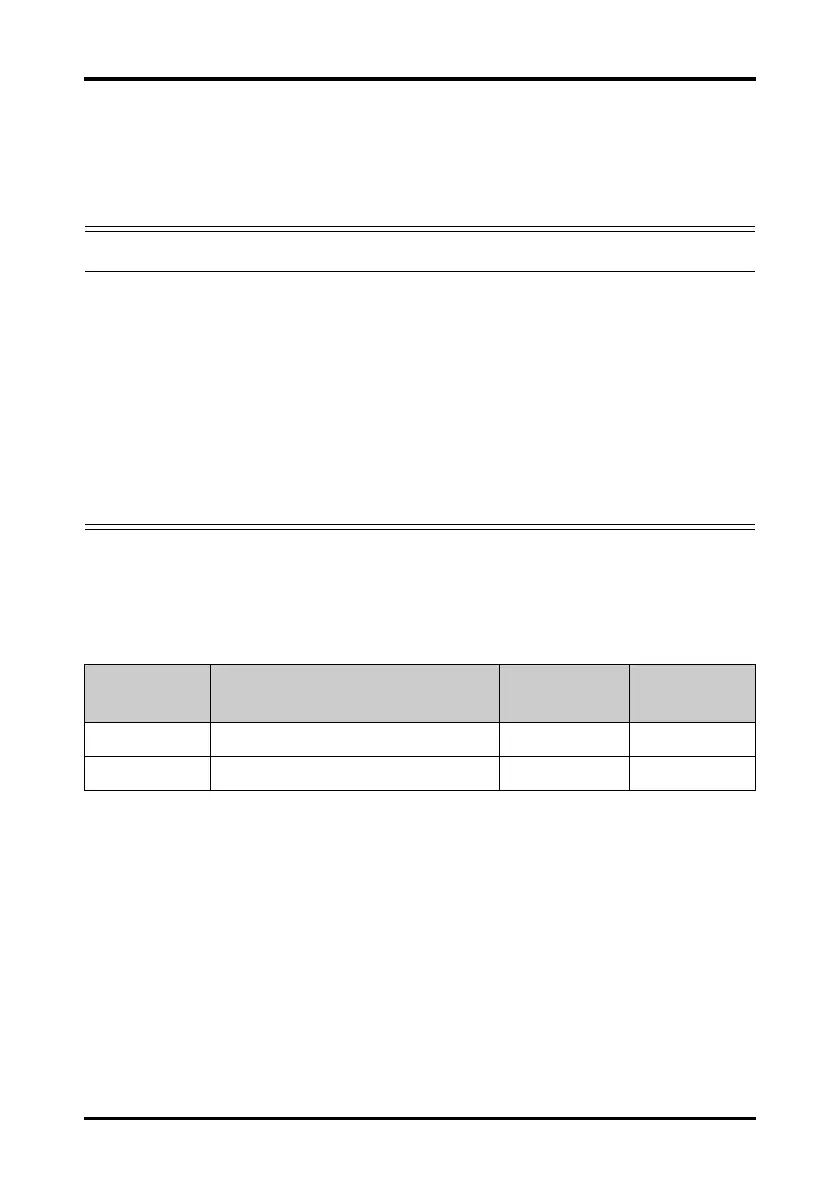
PN Description
Applicable
property
Applicable
patient
0010-10-12304 Adult Electrode (Kendall, package of 10) Disposable Adult
9000-10-07469 Pediatric ECG electrode (3M, package of 50) Disposable Pediatric
 Loading...
Loading...