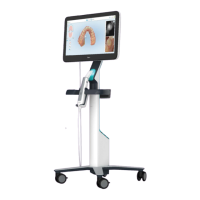
Contraindications
For persons who have been diagnosed with Epilepsy, there is a risk of epileptic shock from the flashing light of the
iTero scanner. These persons should refrain from any eye contact with the flashing light associated with the
system during operation.
Compliance
Class 1 laser compliance
This device complies with 21 CFR 1040.10 and IEC
60825-1.
Safety compliance
This device complies with the following safety standard:
IEC 60601-1 Medical electrical equipment - Part 1:
General requirements for basic safety and essential
performance.
CSA compliance
This device complies with the following CSA standard
for Canada and the USA: UL Std No. 60601-1 – Medical
Electrical Equipment Part 1: General Requirements for
Safety.
EMC compliance
This device complies with the following EMC standard:
IEC 60601-1-2 Medical electrical equipment - Part 1-2:
General requirements for basic safety and essential
performance - Collateral standard: Electromagnetic
phenomena - Requirements and tests.
ANATEL compliance
This device complies with ANATEL resolution nº
242/2000 under the number ANATEL 02563-15-06534.
FCC compliance
This device complies with Part 15 of FCC Rules and its
operation is subject to the following two conditions:
1. This device may not cause harmful interference.
2. This device must accept any interference received,
including interference that may cause undesired
operation.
FCC warning
Modifications to the device that are not expressly
approved by the manufacturer may void your authority
to operate the device under FCC Rules.
iTero Element 5D and iTero Element 5D Plus imaging systems User manual
ii © 2022 Align Technology, Inc. All rights reserved.
 Loading...
Loading...