Instructions for use Primus Infinity Empowered SW 4.5n 273
Technical data
Electromagnetic immunity
The medical device is intended for use in an elec-
tromagnetic environment as specified below. The
user must ensure that the medical device is used in
such an environment.
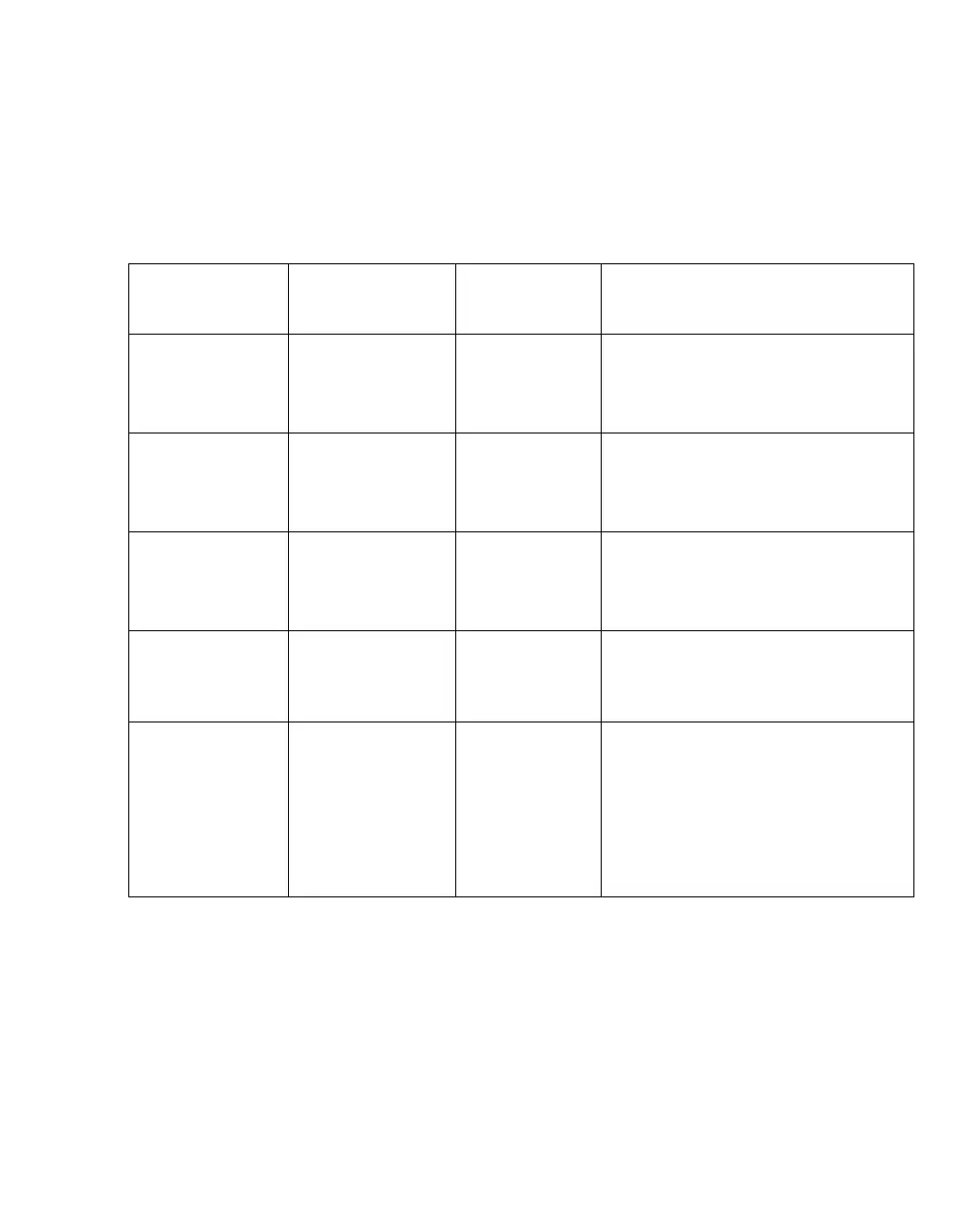
Immunity against IEC 60601-1-2 test
level
Compliance
level (medical
device)
Electromagnetic environment
Electrostatic dis-
charge, ESD
(IEC 61000-4-2)
Contact discharge:
±6 kV
±6 kV Floors should be wood, concrete, or ce-
ramic tiles. If floors are covered with syn-
thetic material, the relative humidity
should be at least 30 %.
Air discharge: ±8 kV ±8 kV
Electrical fast tran-
sients / bursts
(IEC 61000-4-4)
Power supply lines:
±2 kV
±2 kV Mains voltage quality should be that of a
typical commercial or hospital environ-
ment.
Longer input lines/
output lines: ±1 kV
±1 kV
Surges on AC
mains lines
(IEC 61000-4-5)
Common mode:
±2 kV
±2 kV Mains voltage quality should be that of a
typical commercial or hospital environ-
ment.
Differential mode:
±1 kV
±1 kV
Power frequency
magnetic field 50/
60 Hz
(IEC 61000-4-8)
3 A/m 3 A/m No equipment with extraordinarily strong
power frequency magnetic fields (power
transformers, etc.) should be operated in
close vicinity to the medical device.
Voltage dips and
short interruptions
on AC mains input
lines
(IEC 61000-4-11)
Dip >95 %,
0.5 periods
>95 %,
0.5 periods
Mains voltage quality should be that of a
typical commercial or hospital environ-
ment. If the user of the medical device
requires continued operation during
mains power supply interruptions, it is
recommended that the medical device is
powered from an uninterruptible power
supply or a battery.
Dip 60 %, 5 periods 60 %, 5 periods
Dip 30 %, 25 periods 30 %, 25 periods
Dip >95 %,
5 seconds
>95 %,
5 seconds
 Loading...
Loading...