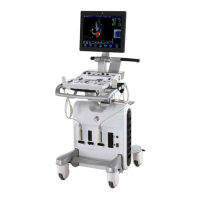
GE
P
ART NUMBER FN091065, REVISION 2 VS5 N AND VS6 N SERVICE MANUAL
10-20 Section 10-6 - Electrical Safety Tests
PRELIMINARY
Section 10-6
Electrical Safety Tests
10-6-1 Safety Test Overview
The electrical safety tests in this section are based on and conform to NFPA 99 (For USA) and IEC
60601-1 Medical Equipment Safety Standards. They are intended for the electrical safety evaluation of
cord-connected, electrically operated, patient care equipment. If additional information is needed, refer
to the NFPA 99 (for USA) and IEC 60601-1 documents.
Test the system, peripherals and probes for current leakage.
To minimize the risk of a probe causing electrical shock, the customer should observe the following
recommendations:
• Do not use a probe that is cracked or damaged in any way
• Check probe current leakage, as shown below:
THE USER MUST ENSURE THAT THE SAFETY INSPECTIONS ARE PERFORMED AT
LEAST EVERY 12 MONTHS ACCORDING TO THE REQUIREMENTS OF THE PATIENT
SAFETY STANDARD IEC-EN 60601-1. ONLY TRAINED PERSONS ARE ALLOWED TO
PERFORM THE ABOVE-MENTIONED SAFETY INSPECTIONS.
COMPARE ALL SAFETY-TEST RESULTS WITH SAFETY-TEST RESULTS OF PREVIOUSLY
PERFORMED SAFETY TESTS E.G. LAST YEAR ETC. IN EVENT OF UNEXPLAINABLE ABRUPT
CHANGES OF SAFETY-TEST RESULTS, CONSULT EXPERIENCED AUTHORIZED SERVICE
PERSONNEL OR GE FOR FURTHER ANALYSIS.
To avoid the risk of electrical shock, the unit under test must not be connected to other electrical
equipment. Remove all interconnecting cables and wires. The operator and patient must not
come into contact with the unit while the tests are being performed.
Possible risk of infection. Do not handle soiled or contaminated probes and other components
that have been in patient contact. Follow appropriate cleaning and disinfecting procedures
before handling the equipment.
EXCESSIVE CURRENT LEAKAGE CAN CAUSE FATAL INJURY. EXCESSIVE CURRENT
LEAKAGE CAN INDICATE DEGRADATION OF INSULATION OR OTHER PART AND
COULD POTENTIALLY CAUSE ELECTRICAL FAILURE. DO NOT USE PROBES OR
EQUIPMENT THAT HAVE EXCESSIVE CURRENT LEAKAGE.
Probe Frequency for Checking
Surface
Once per year
(or in accordance with QA program)
Endocavitary
Twice per year
(or in accordance with QA program)
Any probe
Whenever damage is suspected
 Loading...
Loading...