Pathway Technical Reference Manual
Proprietary 136 of 190
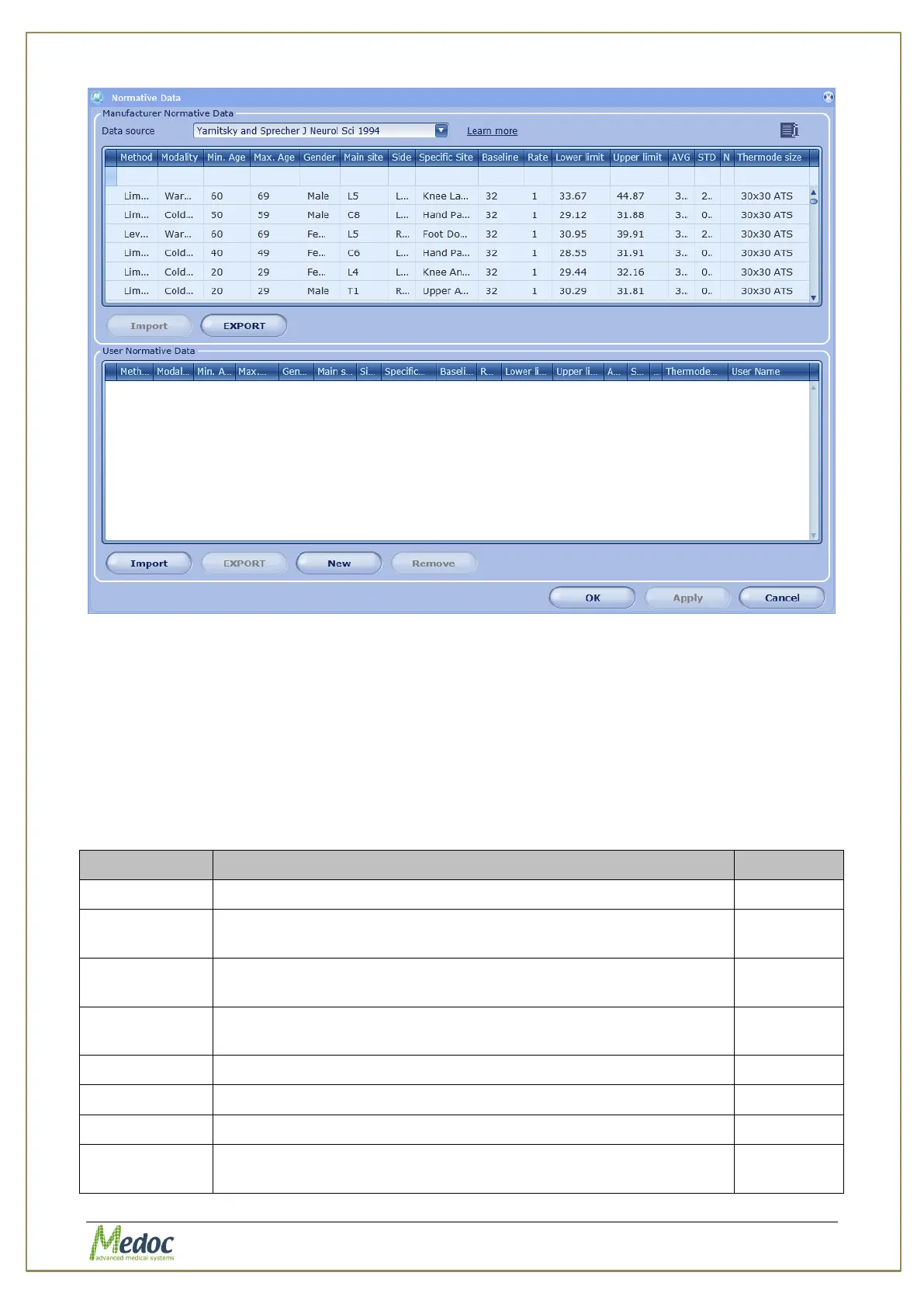
Figure 94: Normative Data Editor Screen
The Normative Data Editor screen is divided into two sections:
1. Manufacturer Normative Data
2. User Normative Data
The Manufacturer Normative Data table is a ‘read-only’ table. The data cannot be edited or
modified. If needed, you can export the data, modify it and upload it again as User Normative
Data. To add customized normative data use the User Normative Data table.
Normative data includes the following parameters:
Table 33: Normative Data Fields
The test method (Limits, Levels, …)
The test modality (Cold Detection Threshold, Warm Detection,
…)
Lower limit of age for which this Normative Data is applicable
Upper limit of age for which this Normative Data is applicable
The gender that this Normative Data is applicable
The dermatome that was used to collect the normative data.
The anatomic side that was used to collect normative data.
The specific body site that was used to collect the normative
data.
 Loading...
Loading...