Chapter 1 - Introduction Important Safety Information
6 Apollo Operator’s Manual
Part Number: 90 38 237, 6th edition
Safe Connection with Other Electrical
Equipment
Systems must meet the requirements in accordance
with IEC/EN 60601-1-1 and IEC/EN 60601 -1-2.
General information on electromagnetic compatibility
(EMC) according to the international EMC standard
IEC 60601-1-2: 2001
Medical electrical equipment needs special
precautions regarding electromagnetic compatibility
(EMC) and needs to be installed and put into service
according to the EMC information provided in the
technical documentation available from DrägerService
upon request.
Portable and mobile RF communications equipment
can affect medical electrical equipment.
Pins of connectors identified with the ESD
warning symbol shall not be touched and not
be connected unless ESD precautionary
procedures are used. Such precautionary
procedures may include anti-static clothing and shoes,
the touch of a ground stud before and during
connecting the pins, or the use of electrically isolating
and anti-static gloves. All staff involved in the above
shall receive instruction in these procedures.
Indications for Use
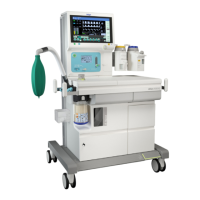
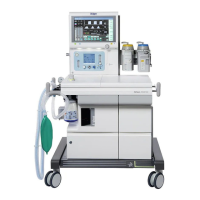
The Apollo is indicated as a continuous flow
anesthesia system. The Apollo may be used for
manually assisted or automatic ventilation, delivery of
gases and anesthetic vapor, and monitoring of oxygen
and CO
2
concentration, breathing pressure, respiratory
volume, and anesthetic agent concentration and
identification.
.
Intended Use
The Apollo is an inhalation anesthesia machine for use
in operating, induction, and recovery rooms. It can be
used with rebreathing systems, semi-closed to virtually
closed systems with low flow and minimal flow
techniques, and non-rebreathing systems (with the
Auxiliary Common Gas Outlet).
It may be used with O
2, N2O, and air supplied by a
medical gas pipeline system or by externally mounted
gas cylinders. Anesthetic agent can be delivered via
vaporizers mounted on the machine.
The Apollo is equipped with a compact breathing
system, providing fresh gas decoupling, PEEP, and
pressure limitation. It has an electrically driven and
electronically controlled ventilator.
The following ventilation modes are available:
• Volume Controlled Ventilation, with activation of
synchronization, pressure support (optional)
• Pressure Controlled Ventilation, with activation of
synchronization, pressure support (optional)
• Manual Ventilation
• Spontaneous Breathing
• Pressure-Assisted Spontaneous Breathing in
Pressure Support Mode (Optional)
Monitoring is provided for:
• airway pressure
•volume
• inspiratory and expiratory concentrations of O
2
,
N
2
O, CO
2
, and anesthetic agent
•SpO
2
(optional)
Medibus Protocol
Medibus is a software protocol for use in transferring
data between the Apollo and an external medical or
non-medical device (e.g., hemodynamic monitors, data
management systems, or a Windows-based computer
via the RS-232 interface).
.
WARNING !
Electrical connections to equipment not listed in
these Instructions for Use should only be made
following consultations with the respective
manufacturers and shall be in compliance with
national medical device regulations.
CAUTION !
Restriction of Distribution
Federal Law (U.S.) restricts this device to sale by, or
on the order of, a physician.
CAUTION !
Data transferred via Medibus interfaces is for
information only and is not intended as a basis for
diagnosis or therapy decisions.
 Loading...
Loading...