289
Overview of the Omnipod 5 System Pivotal Clinical Study 2525 Overview of the Omnipod 5 System Pivotal Clinical Study
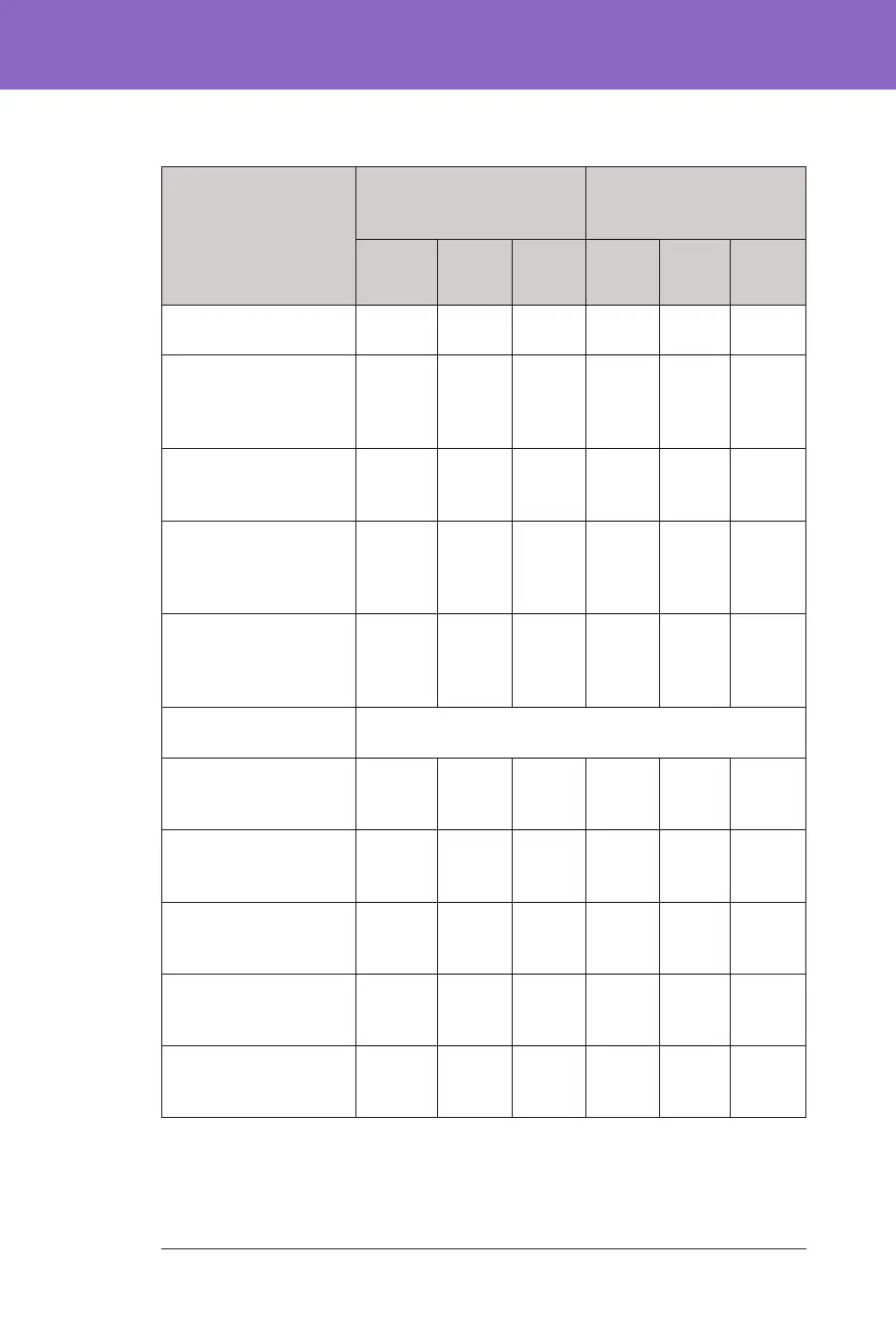
Glycaemic Results Overall (24 hours)
Characteristic
Children (6 to 13.9 years)
(n=112)
Adolescents & Adults (14
to 70 years)
(n=128)
Standard
erapy
Omnip-
od 5
Change Stan-
dard
erapy
Omnip-
od 5
Change
Avg A1C%
(std dev)
7.67%
(0.95%)
6.99%
(0.63%)
-0.71%* 7.16%
(0.86%)
6.78%
(0.68%)
-0.38%*
Avg % time
3.9–10mmol/L,
70–180 mg/dL
(std dev)
52.5%
(15.6%)
68.0%
(8.1%)
15.6%* 64.7%
(16.6%)
73.9%
(11.0%)
9.3%*
Avg sensor glucose,
mmol/L, mg/dL
(std dev)
10.2, 183
(1.8, 32)
8.9, 160
(0.8, 15)
-1.3,
-23*
8.9, 161
(1.6, 28)
8.6, 154
(0.9, 17)
-0.4, -8*
Avg standard deviation
of sensor glucose,
mmol/L, mg/dL
(std dev)
3.8, 68
(0.7, 13)
3.3, 60
(0.6, 10)
-0.5, -9* 3.2, 57
(0.8, 14)
2.7, 49
(0.6, 11)
-0.4, -8*
Avg coecient of
variation of sensor
glucose, %
(std dev)
37.5%
(5.1%)
37.0%
(3.9%)
-0.4% 35.2%
(5.7%)
31.7%
(4.7%)
-3.5%*
% Time in Glucose
Range
Median % <3 mmol/L,
<54 mg/dL
(Q1, Q3)
0.10%
(0.00,
0.41)
0.23%
(0.08,
0.42)
0.04% 0.22%
(0.00,
0.77)
0.17%
(0.06,
0.28)
-0.08%*
Median % <3.9 mmol/L,
<70 mg/dL
(Q1, Q3)
1.38%
(0.42,
2.67)
1.48%
(0.65,
2.23)
0.06% 2.00%
(0.63,
4.06)
1.09%
(0.46,
1.75)
-0.89%*
Avg % >10 mmol/L,
>180 mg/dL
(std dev)
45.3%
(16.7%)
30.2%
(8.7%)
-15.1%* 32.4%
(17.3%)
24.7%
(11.2%)
-7.7%*
Avg % ≥13.9 mmol/L,
≥250 mg/dL
(std dev)
19.1%
(13.1%)
9.6%
(5.4%)
-9.4%* 10.1%
(10.5%)
5.8%
(5.5%)
-4.3%*
Avg % ≥16.7 mmol/L,
≥300 mg/dL
(std dev)
8.5%
(8.9%)
3.5%
(2.9%)
-5.1%* 3.7%
(5.5%)
1.7%
(2.5%)
-2.0%*
Most of the primary and secondary results are presented as averages (avg) with standard deviation
(std dev) values in brackets. Time in range <3.9 mmol/L, <70 mg/dL and <3 mmol/L, <54 mg/dL is
reported as medians with interquartile ranges in brackets (Q1, Q3).
e median is the middle number
in an ascending list of numbers and the interquartile range represents the middle 50% of values.
*Change between the standard-therapy phase and Omnipod 5 System phase was statistically signicant
 Loading...
Loading...