291
Overview of the Omnipod 5 System Pivotal Clinical Study 2525 Overview of the Omnipod 5 System Pivotal Clinical Study
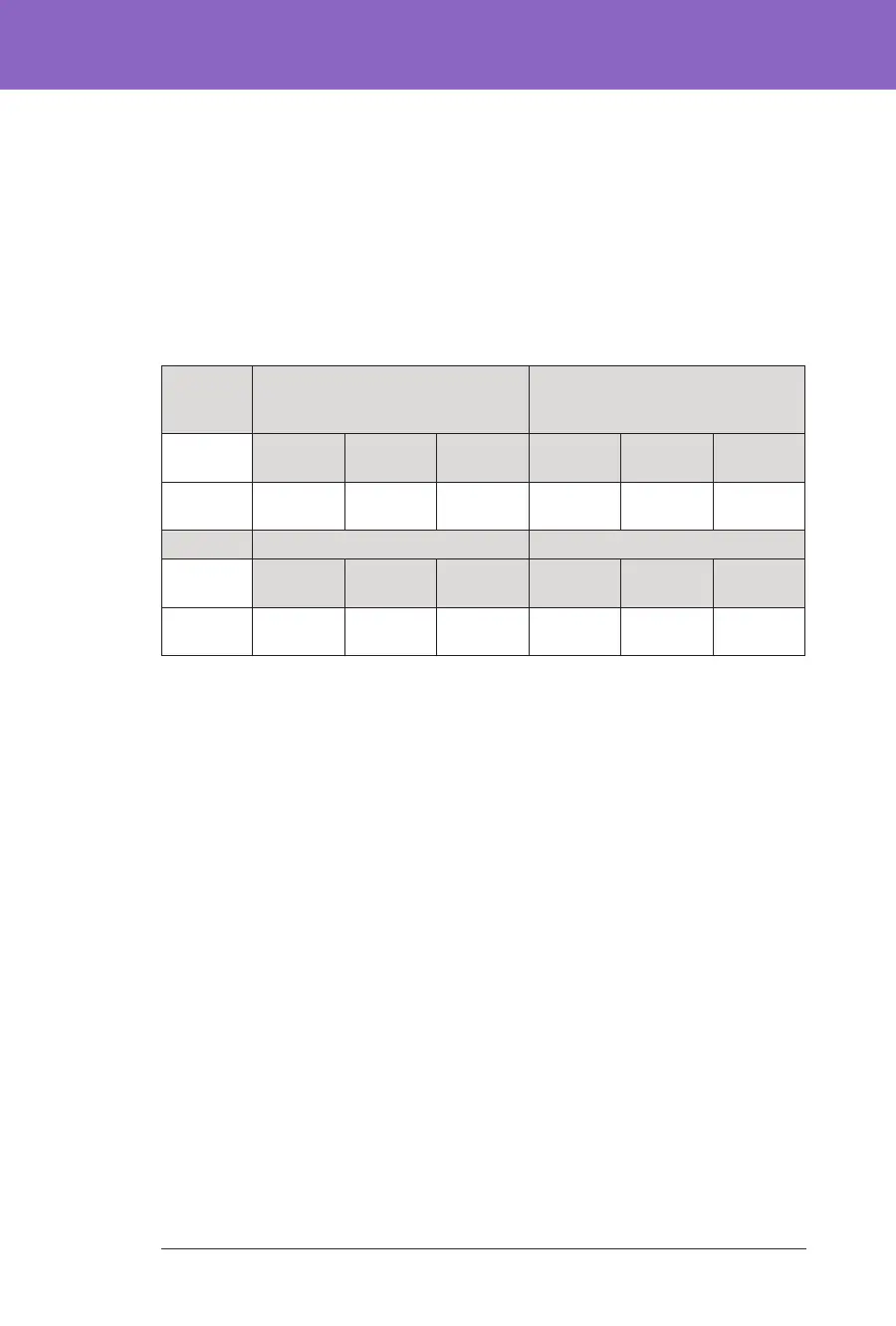
Change in A1C Analysed by Baseline A1C
e table below provides information on the average change in A1C% from
baseline to the end of the 3-month Omnipod 5 System treatment phase, analysed
by baseline A1C% in children (6 to 13.9 years) and adolescents and adults (14
to 70 years). Adolescents, adults and children experienced a reduction in A1C
aer 3 months of Omnipod 5 System use regardless of baseline A1C <8% or ≥8%
category.
Subgroup Analysis of Change in Average A1C(%) by Baseline A1C(%)
Adoles-
cents &
Adults
Baseline A1C <8% (n=105) Baseline A1C ≥8% (n=23)
Baseline
Omnip-
od 5
Change Baseline
Omnip-
od 5
Change
A1C%
(std dev)
‡
6.86%
(0.59%)
6.60%
(0.53%)
-0.27%*
8.55%
(0.42%)
7.63%
(0.67%)
-0.91%*
Children Baseline A1C <8% (n=73) Baseline A1C ≥8% (n=39)
Baseline
Omnip-
od 5
Change Baseline
Omnip-
od 5
Change
A1C%
(std dev)
7.11%
(0.50%)
6.69%
(0.44%)
-0.45%*
8.73%
(0.63%)
7.56%
(0.54%)
-1.18%*
*Change between the standard-therapy phase and the Omnipod 5 System phase was statistically
signicant
‡
Average A1C values are reported with standard deviation values in brackets.
 Loading...
Loading...