Overview of the Omnipod 5 System Pivotal Clinical Study 25
294
25 Overview of the Omnipod 5 System Pivotal Clinical Study
Omnipod 5 System Use
e table below provides information on the average % of time study participants
used the Omnipod 5 System in Automated Mode.
Percentage Time Spent in Automated Mode
Children
(6 to 13.9 years)
n=112
Adolescents & Adults
(14 to 70 years)
n=128
% Time in Automated
Mode
(std dev)
95.2%
(4.0%)
94.8%
(6.0%)
Adverse Events
e table below provides a full list of the adverse events that occurred during
the 3-month Omnipod 5 System treatment phase. ere were 3 severe
hypoglycaemia events not attributable to the Omnipod 5 System automated
insulin delivery or system malfunction, and 1 DKA event from a suspected
infusion-site failure. Other related, but non-glycaemic adverse events included
infection or irritation at infusion site (2 children, 2 adolescents/adults).
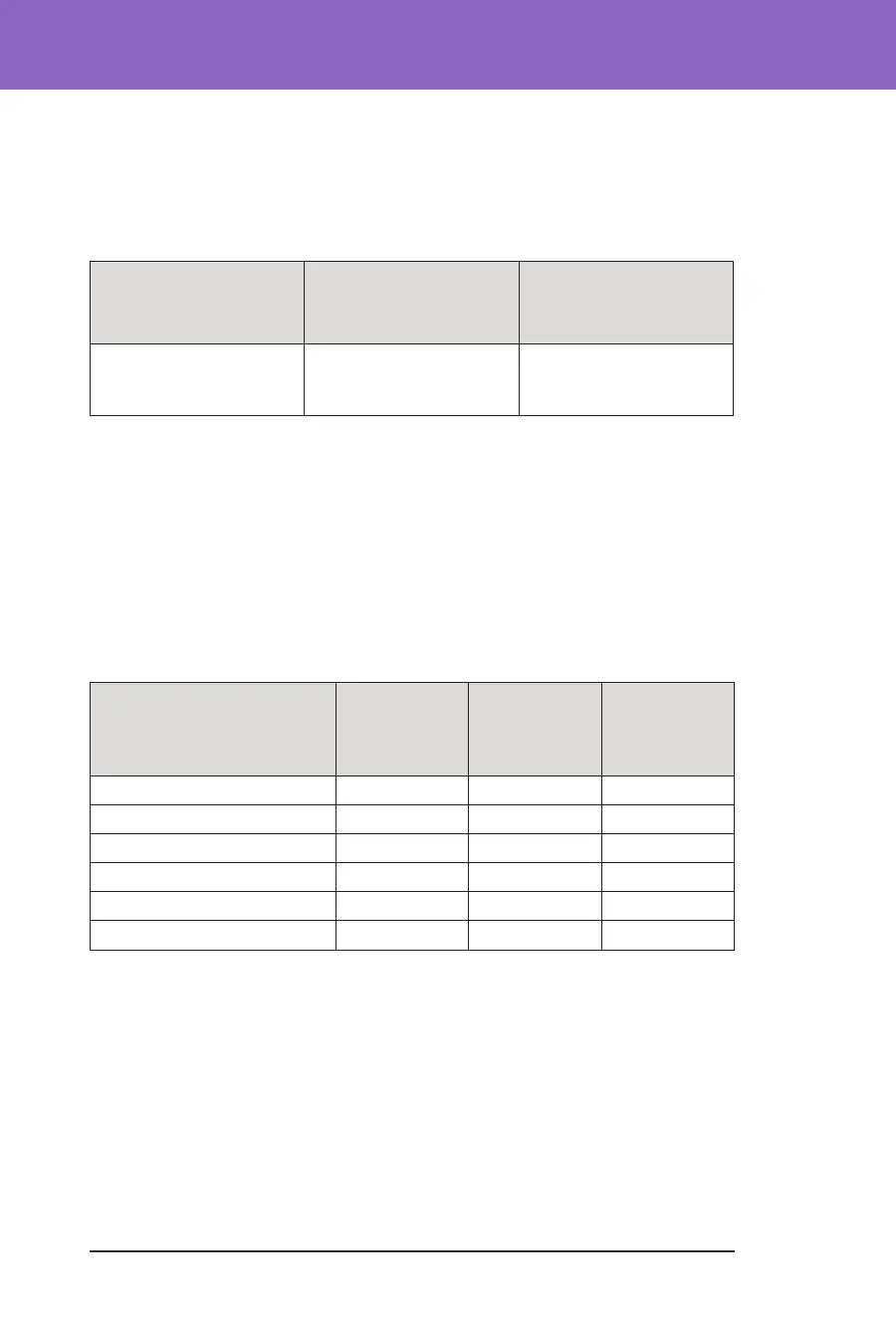
Adverse Events during the Omnipod 5 System Phase
Adverse Event Type Children
(6 to 13.9
years)
(n=112)
Adolescents &
Adults
(14 to 70 years)
(n=128)
Tota l
(6 to 70 years)
(n=240)
Hypoglycaemia
‡
1 0 1
Severe Hypoglycaemia
§
1 2 3
DKA 1 2 1
Hyperglycaemia
‖
1 2 3
Prolonged Hyperglycaemia
**
13 5 18
Other 8 8 16
Results reported as number of events.
‡
Hypoglycaemia resulting in a serious adverse event, but otherwise not meeting the denition of severe
hypoglycaemia.
§
Required the assistance of another person.
‖
Hyperglycaemia requiring evaluation, treatment or guidance from the intervention site, or
hyperglycaemia resulting in a serious adverse event.
**
Meter blood glucose measuring 16.7 mmol/L (≥300 mg/dL) and ketones >1.0 mmol/L
 Loading...
Loading...