305
Overview of the Omnipod 5 System Pivotal Clinical Study 2525 Overview of the Omnipod 5 System Pivotal Clinical Study
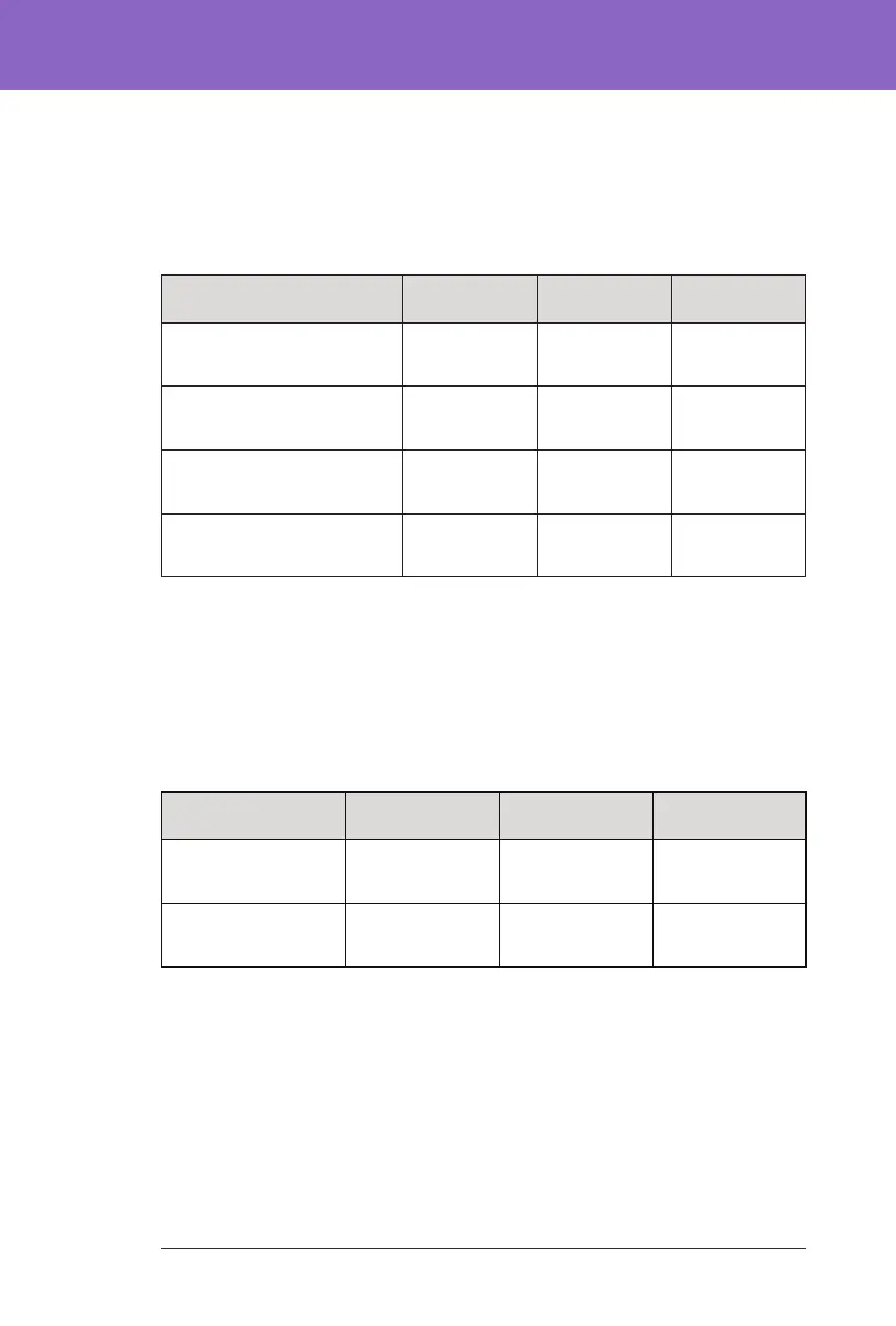
Insulin Requirements
e table below provides information on the average insulin requirements during
the standard-therapy phase and the 3-month Omnipod 5 System phase. Total
daily insulin requirements remained unchanged except for an increase in total
daily basal insulin.
Characteristic
Standard
Therapy
Omnipod 5 Change
Avg total daily insulin (U)
(std dev)
13.7
(4.4)
14.1
(4.0)
0.4
Avg total daily insulin, U/kg
(std dev)
0.69
(0.18)
0.71
(0.15)
0.02
Avg total daily basal insulin,
U/kg, (std dev)
0.28
(0.12)
0.32
(0.10)
0.04*
Avg total daily bolus insulin,
U/kg, (std dev)
0.41
(0.15)
0.39
(0.10)
-0.02
(0.10)
*Change between the standard-therapy phase and the Omnipod 5 System phase was
statistically signicant
Body Mass Index Results
e table below provides information on the average body mass index (BMI) and
BMI z-score during the standard-therapy phase and the 3-month Omnipod 5
System phase. BMI and BMI z-score did not change between the two phases.
Characteristic Standard
Therapy
Omnipod 5 Change
BMI, kg/m2 (std dev)
16.7 (1.5)
16.7 (1.4)
0.1
BMI z-score (std dev) 0.74 (0.95)
0.76 (0.89)
0.05
Omnipod 5 System Use
e median (Q1, Q3) % of time for which study participants used the Omnipod 5
System in Automated Mode was 97.8% (95.8, 98.5).

 Loading...
Loading...