301
Overview of the Omnipod 5 System Pivotal Clinical Study 2525 Overview of the Omnipod 5 System Pivotal Clinical Study
Demographics
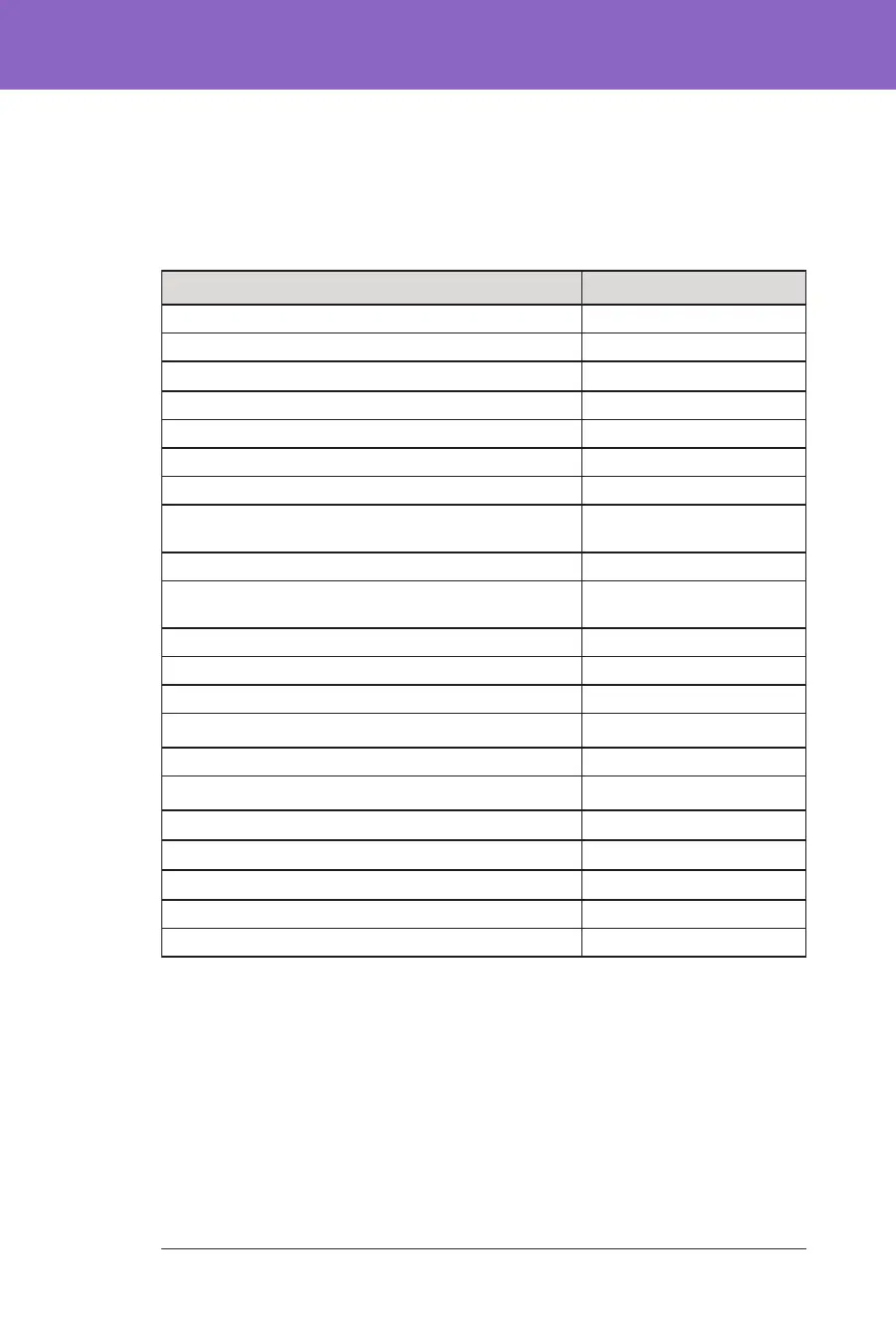
Baseline characteristics, including the demographics of the participants at the start
of the 3-month Omnipod 5 treatment phase, are provided in the table below.
Baseline Characteristics at Omnipod 5 Treatment Phase Start
Characteristic
n 80
Age (years) ± std dev 4.7 ± 1.0
Duration of diabetes (years) 2.3 ± 1.1
A1C§ 7.4% ± 1.0%
Daily insulin dose (U/kg) ¥ 0.69 ± 0.18
Body mass index (BMI) (kg/m
2
) 16.7 ± 1.5
Female sex 34 (42.5%)
Previous¶ or current continuous glucose monitor
(CGM) use
78 (97.5%)
Previous¶ or current pump use 68 (85.0%)
Using multiple daily injection as a standard-therapy
method
12 (15.0%)
Race/Ethnicity‡
White 67 (83.8%)
Hispanic or Latino 5 (6.3%)
Black or African American 4 (5.0%)
Black or African American, White 3 (3.8%)
Asian 3 (3.8%)
Asian, White 2 (2.5%)
Hispanic or Latino 1 (1.3%)
Not Hispanic or Latino 1 (1.3%)
Other (Dominican) 1 (1.3%)
Hispanic or Latino 1 (1.3%)
Plus-minus values are average ± standard deviation; results reported with a number in brackets
aerwards represent the number of participants (% of participants).
§ A1C determined from laboratory assessment.
¥ Baseline total daily insulin dose was determined from data collected during the standard-therapy
phase.
¶ Previous use is dened as having used the device for any duration in the past.
‡ Race and ethnicity were reported by the participants. Groups are not mutually exclusive.
 Loading...
Loading...