Overview of the Omnipod 5 System Pivotal Clinical Study 25
302
25 Overview of the Omnipod 5 System Pivotal Clinical Study
Glycaemic Results
e tables below include information on the primary and secondary glycaemic
results from the standard-therapy phase compared with the 3-month Omnipod 5
System treatment phase. e primary results of the study included change
in average A1C% and % time in range (3.9–10 mmol/L, 70–180 mg/dL).
Participants experienced improvements in A1C and overall time in range aer
3 months of Omnipod 5 System use. is result was achieved with a reduction of
time >10 mmol/L (>180 mg/dL) as well as a reduction in median time
<3.9 mmol/L (<70 mg/dL).
Some limitations to the study include: 1) single-arm design with no control group,
which could lead to an over-estimate of glycaemic improvement; 2) the standard-
therapy phase was shorter than the Omnipod 5 System phase.
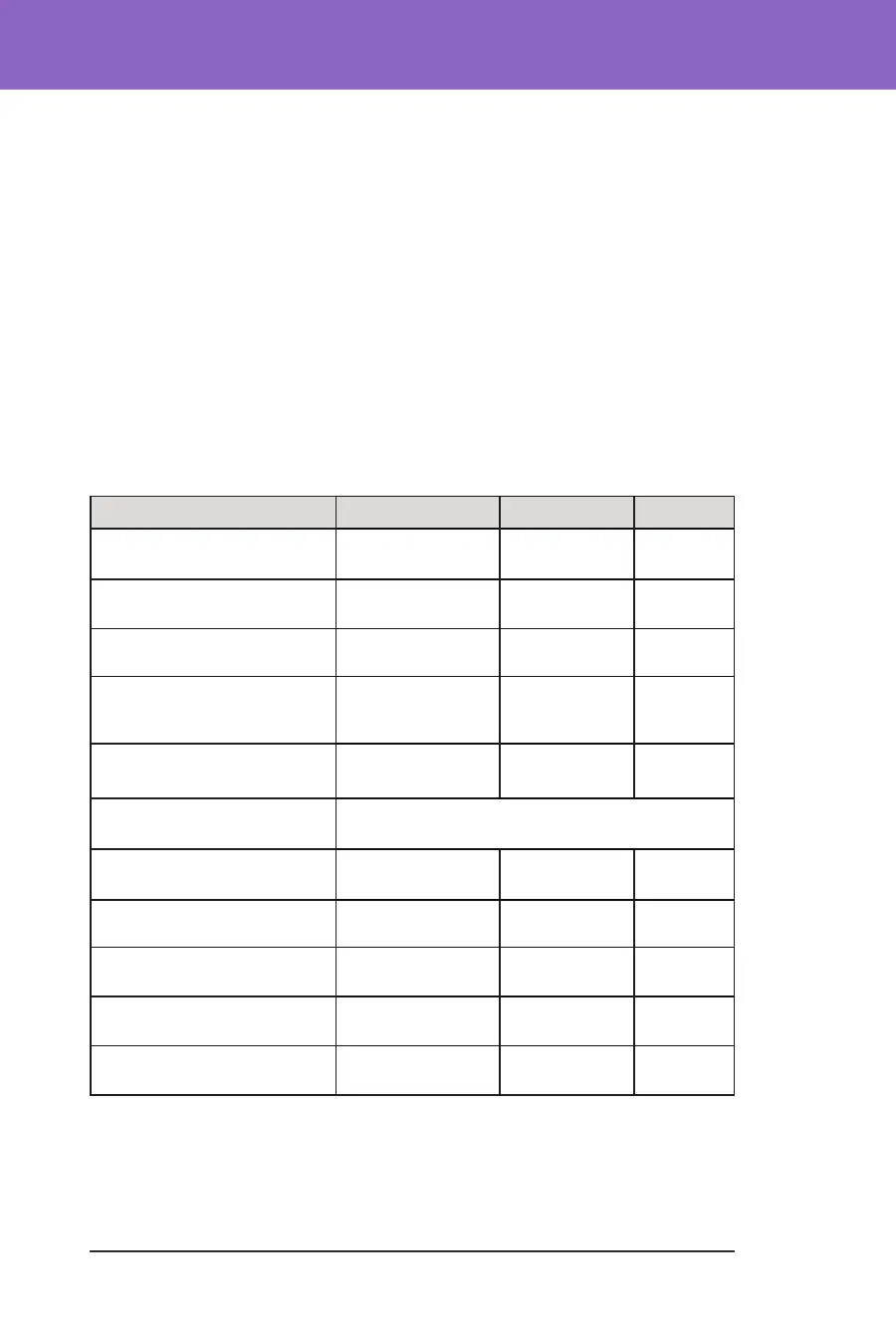
Glycaemic Results Overall (24 hours)
Characteristic Standard Therapy Omnipod 5 Change
Avg A1C%
(std dev)
7.4%
(1.0%)
6.9%
(0.7%)
-0.55%*
Avg % time 3.9–10 mmol/L,
70–180 mg/dL (std dev)
57.2%
(15.3%)
68.1%
(9.0%)
10.9%*
Avg sensor glucose, mmol/L,
mg/dL, (std dev)
9.5, 171.1
(1.7, 30.5)
8.7, 157.4
(0.9, 16.8)
-0.7, -13.7*
Avg standard deviation of
sensor glucose, mmol/L,
mg/dL (std dev)
3.6, 64.9
(0.7, 13.4)
3.3, 59.6
(0.6, 10.3)
-0.3, -5.3*
Avg coefcient of variation of
sensor glucose, % (std dev)
38.1%
(5.5%)
37.7%
(4.0%)
-0.4%
% Time in Glucose Range
Median % <3 mmol/L,
<54 mg/dL (Q1, Q3)
0.24%
(0.05, 0.84)
0.26%
(0.16, 0.60)
0.06%
Median % <3.9 mmol/L,
<70 mg/dL (Q1, Q3)
2.19
(0.89, 4.68)
1.94
(
1.18, 3.43)
-0.27%*
Avg % >10 mmol/L,
>180 mg/dL (std dev)
39.4%
(16.7%)
29.5%
(9.8%)
-9.9%*
Avg % ≥13.9 mmol/L,
≥250 mg/dL (std dev)
14.8%
(12.1%)
9.2%
(5.6%)
-5.6%*
Avg % ≥16.7 mmol/L,
≥300 mg/dL(std dev)
6.0%
(7.3%)
3.2%
(2.8%)
-2.7%*
Most of the primary and secondary results are presented as averages (avg) with standard deviation
(std dev) values in brackets. Time in range <3.9 mmol/L (<70 mg/dL) and <3 mmol/L (<54 mg/dL) is
reported as medians with interquartile ranges in brackets (Q1, Q3). e median is the middle number
in an ascending list of numbers and the interquartile range represents the middle 50% of values.
*Change between the standard-therapy phase and the Omnipod 5 System phase was statistically
signicant
 Loading...
Loading...