295
Overview of the Omnipod 5 System Pivotal Clinical Study 2525 Overview of the Omnipod 5 System Pivotal Clinical Study
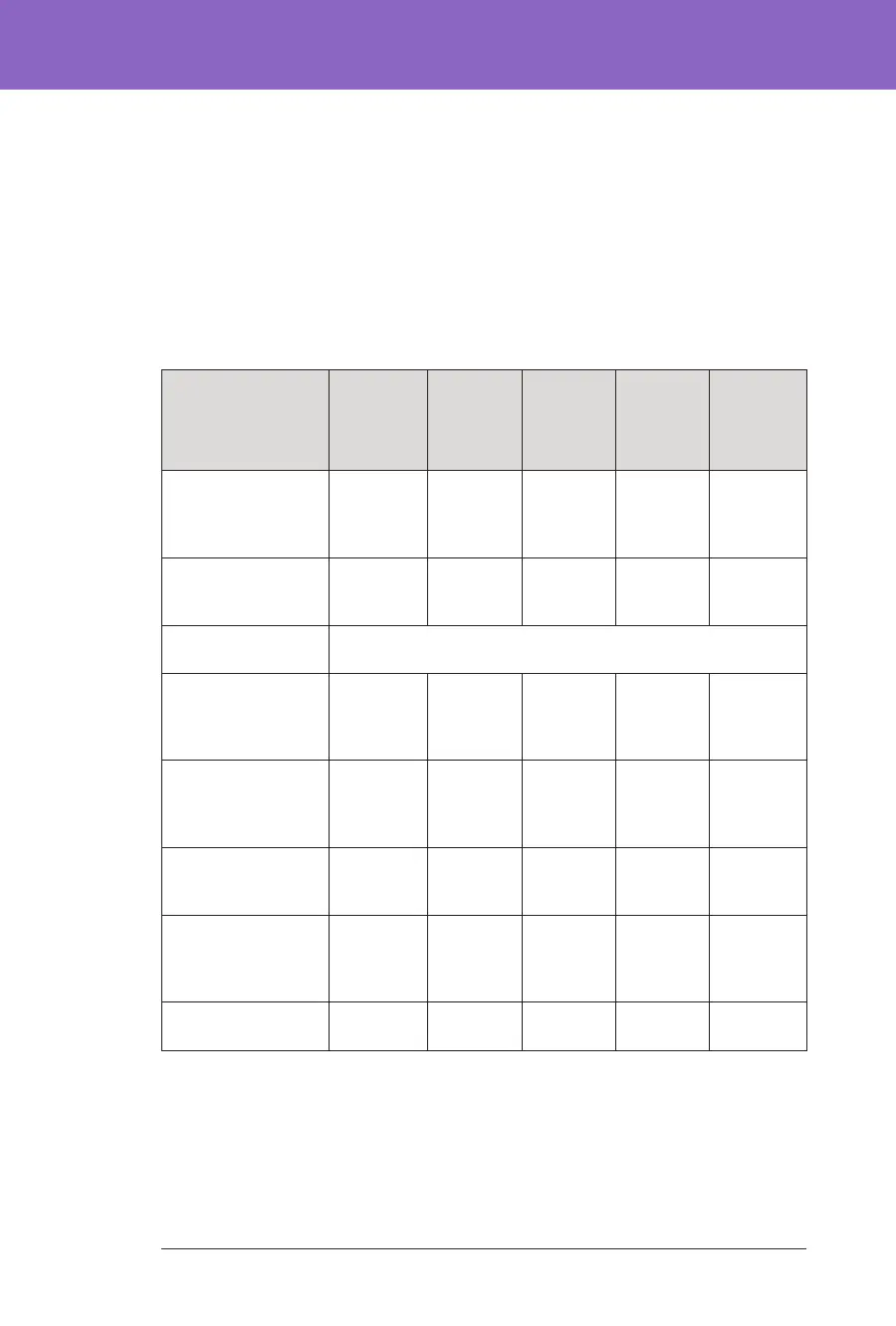
Glycaemic Results at Target Glucose Settings in the Pivotal
Study
e tables below provide information on the glycaemic results at various self-
selected Target Glucose settings during the 3-month Omnipod 5 System phase of
the pivotal study. Of the customisable Glucose targets, the most selected was
6.1 mmol/L (110
mg/dL)
Overall(24 hours)Glycaemic Results at Target Glucose Settings
inChildren(6 to 13.9 years)from the Pivotal Study
Characteristic
6.1 mmol/L,
110 mg/dL
Target
Glucose
(n=98)
6.7 mmol/L,
120 mg/dL
Target
Glucose
(n=74)
7.2 mmol/L,
130 mg/dL
Target
Glucose
(n=47)
7.8 mmol/L,
140 mg/dL
Target
Glucose
(n=12)
8.3 mmol/L,
150 mg/dL
Target
Glucose*
(n=9)
Avg % time
3.9–10 mmol/L,
70–180 mg/dL
(std dev)
68.4%
( 9.1%)
67.5%
(9.7%)
64.2%
(14.3%)
59.2%
(16.9%)
53.3%
(18.2%)
Avg sensor glucose,
mmol/L, mg/dL
(std dev)
8.8, 159
(0.9, 17)
9.1, 163
(0.9, 16)
9.4, 169
(1.3, 24)
9.9, 178
(1.3, 24)
10.2, 183.6
(1.3, 23.9)
% Time in glucose
range
Median %
<3 mmol/L,
<54 mg/dL
(Q1, Q3)
0.22%
(0.06, 0.49)
0.18%
(0.05,
0.33)
0.09%
(0.00,
0.21)
0.04%
(0.00,
0.34)
0.00%
(0.00, 0.00)
Median %
<3.9 mmol/L,
<70 mg/dL
(Q1, Q3)
1.51%
(0.76, 2.38)
1.16%
(0.58,
1.94)
0.71%
(0.26,
1.63)
0.59%
(0.05,
1.52)
0.12%
(0.00, 0.21)
Avg % >10 mmol/L,
>180 mg/dL
(std dev)
29.7%
(9.6%)
31.1%
(10.0%)
34.5%
(14.8%)
39.9%
(16.6%)
46.4%
(18%)
Avg %
≥13.9 mmol/L,
≥250 mg/dL
(std dev)
9.7%
(5.8%)
10.0%
(6.3%)
11.8%
(9.0%)
14.6%
(11.1%)
13.3%
(11.9%)
Cumulative number
of person-days
6,289 2,716 941 99 73

 Loading...
Loading...