297
Overview of the Omnipod 5 System Pivotal Clinical Study 2525 Overview of the Omnipod 5 System Pivotal Clinical Study
Omnipod 5 System Pre-Pivotal Glycaemic Results at Target
Glucose Settings
Glycaemic Results at Target Glucose Settings in the Pre-Pivotal Study
e goal of the pre-pivotal study of the Omnipod 5 System was to assess
the safety and ecacy of the system. is single-arm, multicentre,
prospective study enrolled 18 children (6 to 13.9 years) and 18 adults
(14 to 70 years) with type 1 diabetes. A 2-week standard-therapy phase
(usual insulin regimen) was followed by 2 weeks of Omnipod 5 System use
in Automated Mode. e 2-week Omnipod 5 phase included 3 days
of required use at each of the Target Glucose settings of 7.2 mmol/L
(130 mg/dL), 7.8 mmol/L (140 mg/dL) and 8.3 mmol/L (150 mg/dL) for a
total of 9 days, followed by 5 days of free choice of Target Glucose ranging
from 6.1–8.3 mmol/L (110–150 mg/dL).
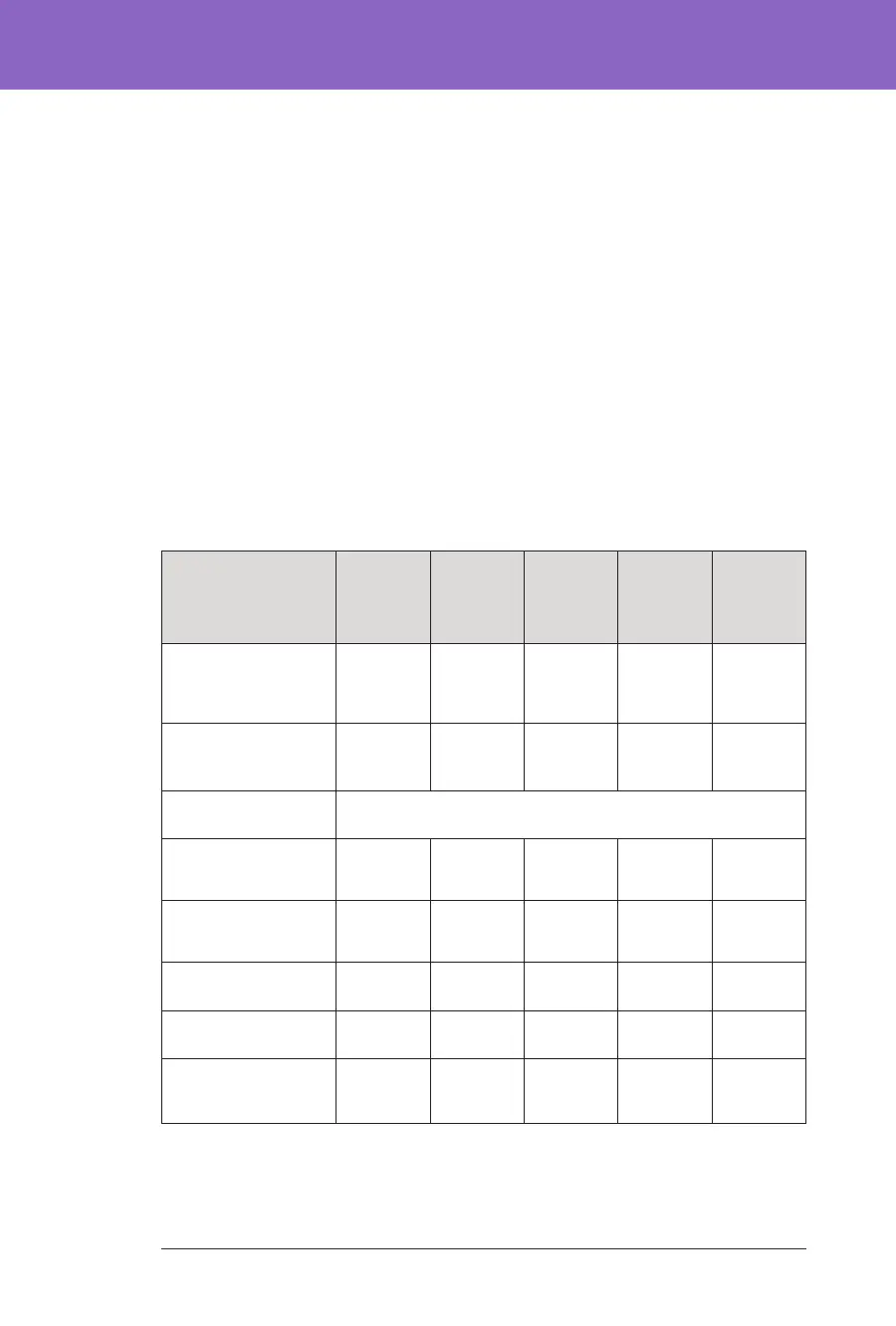
Overall (24 hours) Glycaemic Results at Target Glucose Settings in
Children (6 to 13.9 years) from the Pre-Pivotal Study
Characteristic
6.1 mmol/L,
110 mg/dL
Target
Glucose
(n=11)
6.7 mmol/L,
120 mg/dL
Target
Glucose
(n=3)
7.2 mmol/L,
130 mg/dL
Target
Glucose
(n=18)
a
7.8 mmol/L,
140 mg/dL
Target
Glucose
(n=18)
8.3 mmol/L,
150 mg/dL
Target
Glucose
(n=18)
b
Avg % time
3.9–10 mmol/L,
70–180 mg/dL
(std dev)
71.2%
(10.2%)
66.8%
(12.9%)
61.5%
(7.7%)
64.8%
(11.6%)
53.5%
(11.0%)
Avg sensor glucose,
mmol/L, mg/dL
(std dev)
8.6, 155.2
(1.0, 18.2)
9.4, 170
(0.9, 16)
9.7, 174.1
(0.6, 11.4)
9.6, 172.7
(1.0, 17.2)
10.2, 182.9
(0.9, 15.3)
% Time in glucose range
Median %
<3 mmol/L,
<54 mg/dL (Q1, Q3)
0.1%
(0.0, 0.4)
0.2%
(0.0, 0.3)
0.0%
(0.0, 0.3)
0.0%
(0.0, 0.0)
0.0%
(0.0, 0.1)
Median %
<3.9 mmol/L,
<70 mg/dL (Q1, Q3)
0.9%
(0.4, 2.8)
0.3%
(0.2. 2.2)
0.5%
(0.1, 0.8)
0.1%
(0.0, 0.5)
0.5%
(0.0, 0.8)
Avg % >10 mmol/L,
>180 mg/dL (std dev)
27.1%
(11.4%)
32.3%
(11.9%)
37.7% (7.9) 34.6%
(12.1%)
45.9%
(11.0%)
Avg % ≥13.9 mmol/L,
≥250 mg/dL (std dev)
6.8% (6.3%) 14.4%
(6.2%)
13.2%
(5.8%)
10.6%
(7.3%)
12.8%
(8.1%)
Cumulative number of
person-days
47.7 8.7 73.3 56.3 61.5
a
All participants initiated the system at the 7.2 mmol/L (130 mg/dL) Target Glucose for 3 days.
b
e glycaemic results at the 8.3 mmol/L (150 mg/dL) Target Glucose setting include times with the
Activity feature ON and OFF, meaning the results recorded during this time may include those when
participants felt their insulin needs were reduced.
 Loading...
Loading...