Instrument verification description
Purpose
Description Pass criteria
Confirms the
performance of the
instrument.
Quantifies the number of copies of
the human RNase P gene in samples
with known concentrations of the
corresponding DNA template.
The instrument passes performance specifications
if the following inequality is true and the
instrument successfully distinguishes between
unknown populations A and B with a statistical
confidence level of 99.7%.
[(C
qA
) – 3(σ
CqA
)] > [(C
qB
) + 3(σ
CqB
)]
where:
•
C
qA
= Average C
q
of unknown population A
•
σ
CqA
= Standard deviation of unknown
population A
•
C
qB
= Average C
q
of unknown population B
•
σ
CqB
= Standard deviation of unknown
population B
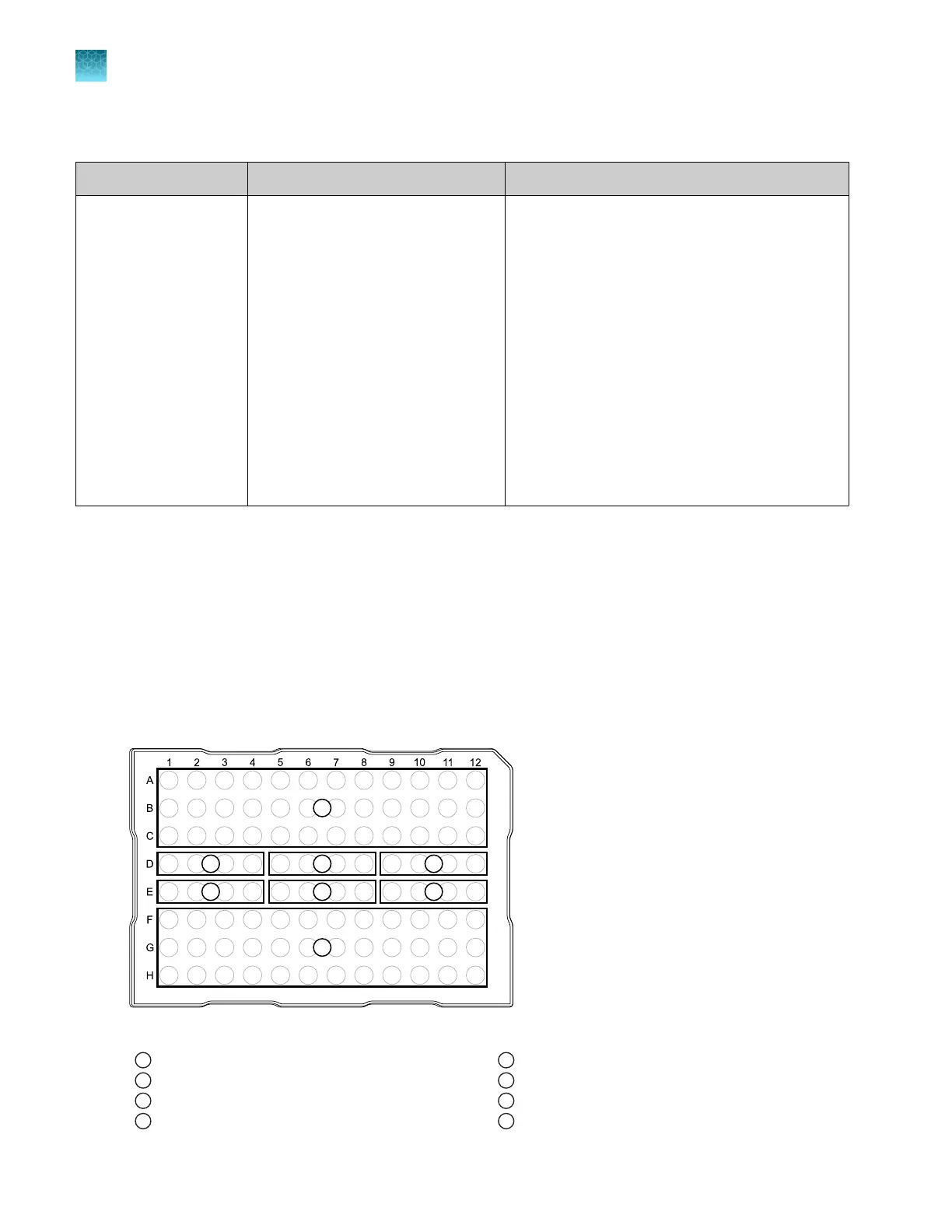
RNase P instrument verification plate
The RNase P plate contains the reagents necessary for the detection and quantitation of genomic
copies of the human RNase P gene (a single-copy gene encoding the RNase moiety of the RNase P
enzyme). Each well contains the following components:
•
PCR Mast
er Mix
•
RNase P primers
•
A FAM
™
dye-labeled probe
•
A known concentration of human genomic DNA template
Figure 13 96-well RNase P plate
1
Unknown A (5,000)
2
NTC (no template control)
3
STD 1,250 copies
4
STD 2,500 copies
5
STD 5,000 copies
6
STD 10,000 copies
7
STD 20,000 copies
8
Unknown B (10,000)
Chapter 7 Calibrate and verify instrument performance
Perform verification using RNase P plates
7
104
QuantStudio
™
6 Pro Real-Time PCR System and QuantStudio
™
7 Pro Real-Time PCR System User Guide

 Loading...
Loading...