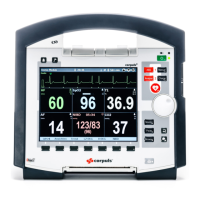
Why is the QRS tone inaudible on GS corpuls3 Medical Equipment?
- AAndrea MckeeSep 23, 2025
Acoustic reproduction of the QRS tone may not be enabled. Press the left softkey [QRS] in monitoring mode. Also, the loudspeaker of the monitoring unit may not be enabled. Activate the loudspeaker of the monitoring unit.