GEDRAFT LOGIQ P9/P7
D
IRECTION 5604324, REVISION 11 DRAFT (JANUARY 24, 2019) SERVICE MANUAL
Chapter 4 - Functional Checks 4-5
4-3-1-2 Appearance Inspection
* Required for Asia.
1) Check presence of LCD Caution Label.
2) Check presence of Gender Caution Label.l
NOTE: Some countries may not have "gender determination" label.
3) Check presence of Multi Caution Label.
4) Check presence of Rating Plate.
NOTE: Rating Plate design slightly differ depending on Console destination. At minimum simply check Console
number, manufacturing date and serial Number.
5) Check presence of UL Label
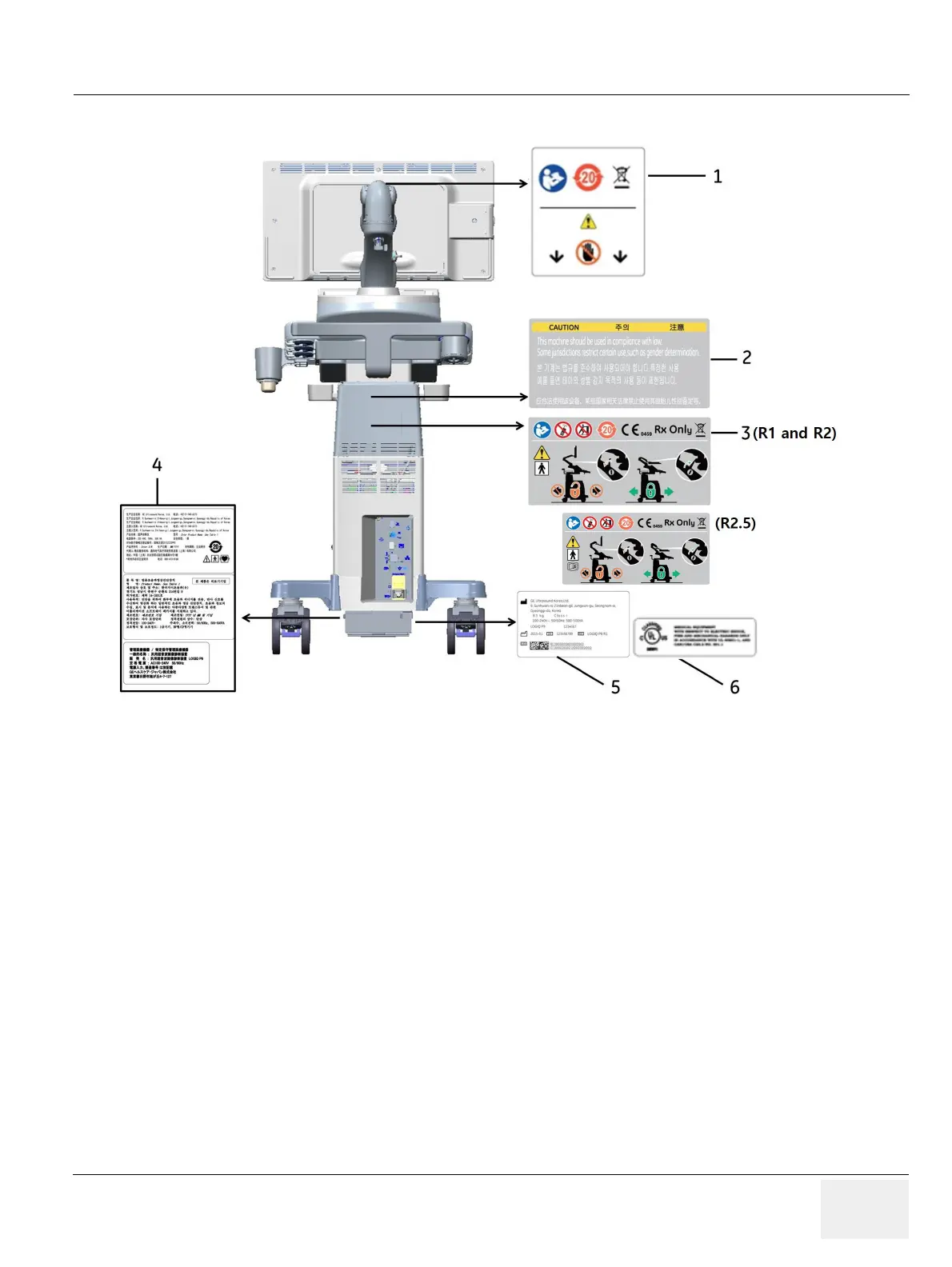
Figure 4-2 Label Location (R1, R2, and R2.5)
1. LCD CAUTION LABEL
2. GENDER CAUTION LABEL (ONLY FOR INDIA, CHINA, KOREA)
3. MULTI CAUTION LABEL
4.
LOGIQ P9/P7 RATING LABEL (FOR CHINA, FOR KOREA, FOR
JAPAN)
5.
LOGIQ P9/P7 RATING LABEL
6. UL LABEL
 Loading...
Loading...