GEDRAFT LOGIQ P9/P7
D
IRECTION 5604324, REVISION 11 DRAFT (JANUARY 24, 2019) SERVICE MANUAL
Chapter 1 - Introduction 1-11
For hardware installation procedures see: Chapter 3 - Connection of Auxiliary Devices, on page 3-11.
!! CAUTION:
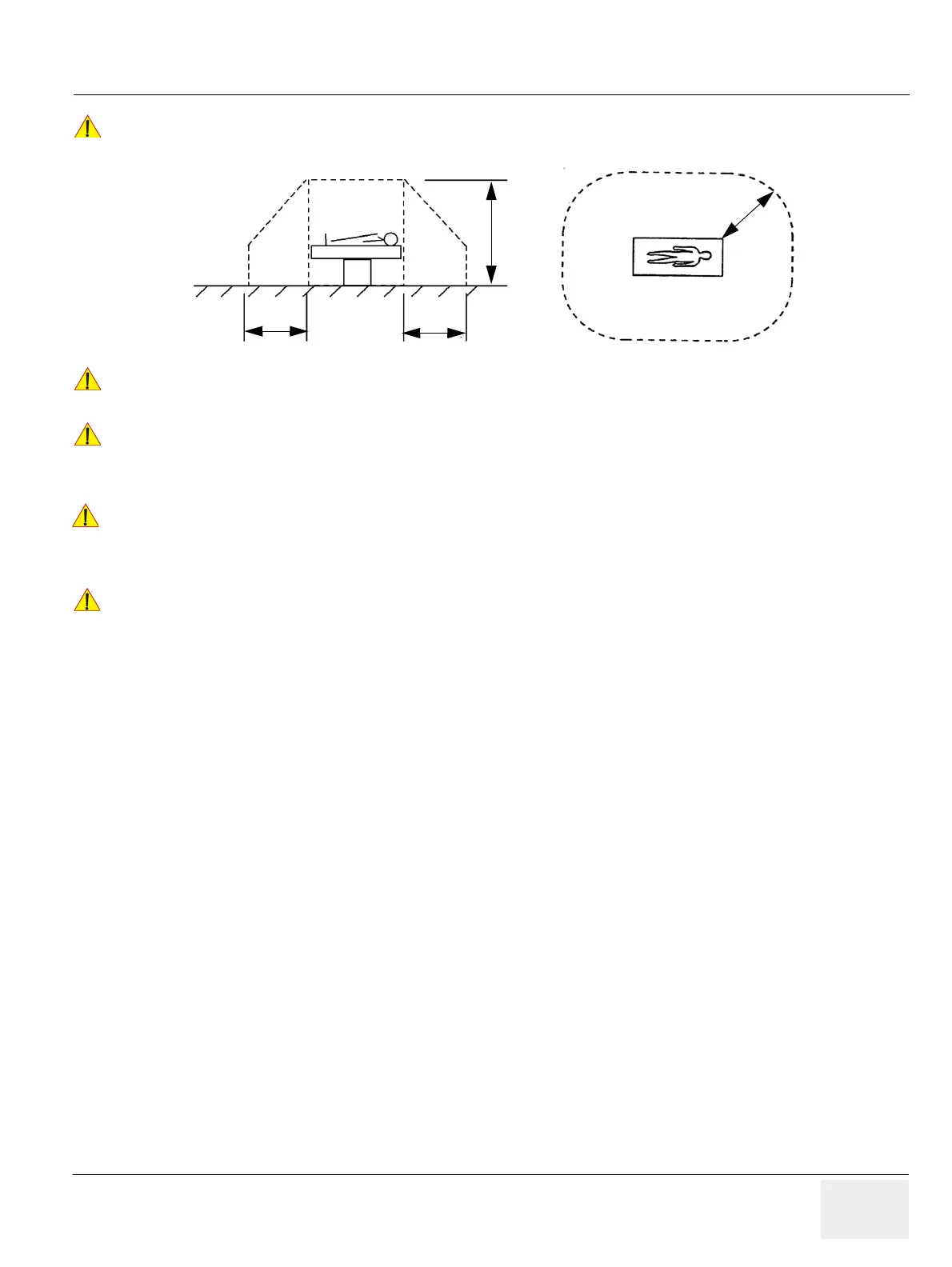
Please observe that some printers may not be medical devices! If the Bluetooth Printer and/or
Line Printers are no medical devices, they have to be located outside of the patient environment
(according to IEC 60601-1 / ANSI/AAMI ES60601-1).
!! CAUTION:
Auxiliary equipment must only be connected to the main console with the special main outlet
provided for the electrical safety of the system.
!! CAUTION:
Auxiliary equipment with direct main connection requires galvanic separation of the signal and/
or control leads.
!! WARNING:
After each installation, the leakage currents have to be measured according to
IEC 60601-1 respectively ANSI/AAMI ES60601-1.
!! NOTICE:
All peripherals mounted on the LOGIQ P9/P7 system chassis must be firmly secured in position.
 Loading...
Loading...