CAPINTEC, INC. CAPTUS
®
4000e
5-14 QUALITY ASSURANCE August 17
6. Place the Cs137 source rod in the selected detector. Since the Constancy Test is a
measure of reproducibility, it is important that the source be placed in exactly the same
position each time the Constancy Test is performed.
7. Select the OK button to proceed with the test, or else select the Cancel button to stop the
process. As the count progresses, the live spectra and measurement time are displayed.
8. To stop the counting, select the Abort button or press Alt+A. The collected data is
discarded. To re-start the process, select the Start Procedure button or press Alt+S.
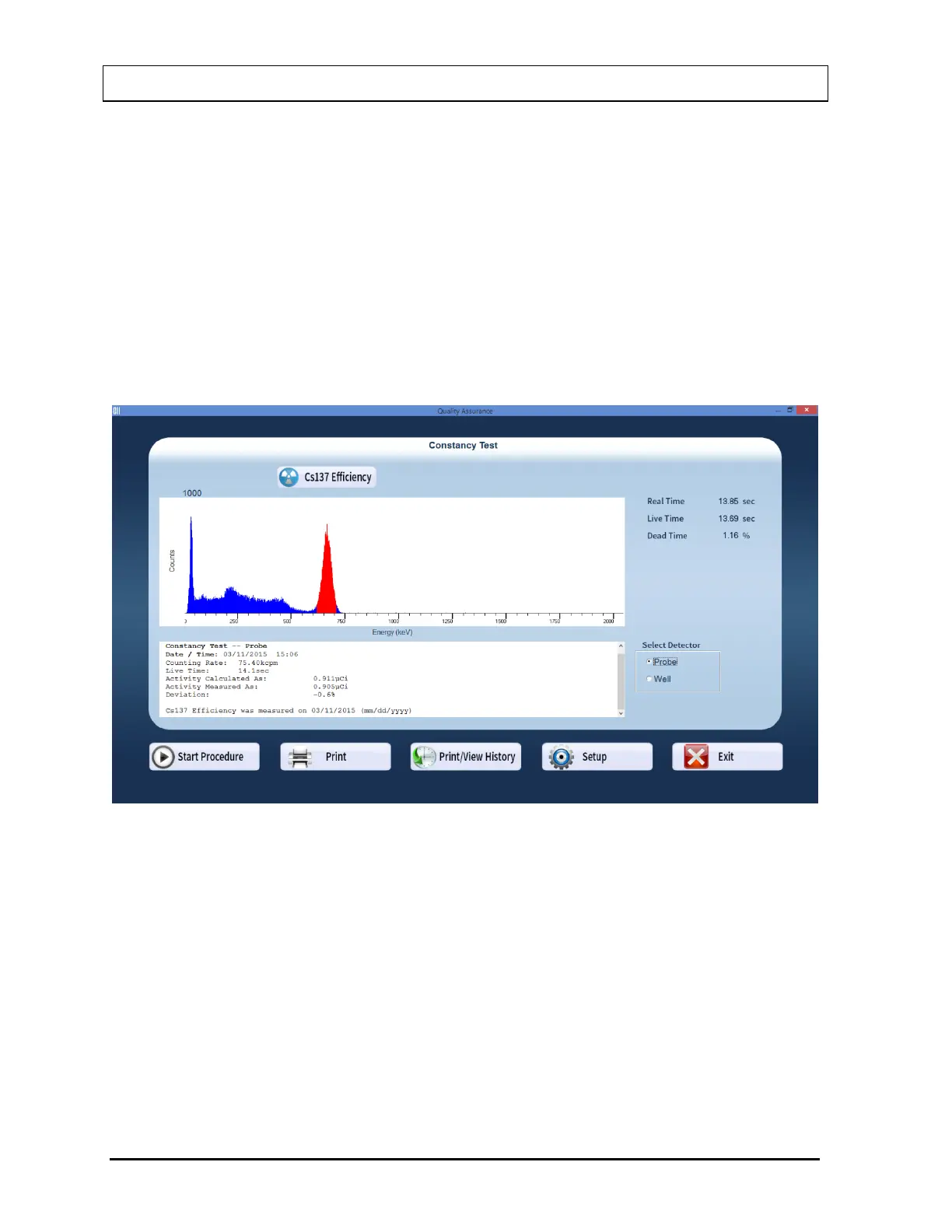
9. After the test is completed, the results of the test appear in the box below the
measurement spectrum as shown in Figure 5-15 Constancy Test Results Screen. Note
the displayed Deviation value.
Figure 5-15 Constancy Test Results Screen
An unacceptable deviation is indicated in red as HIGH.
If the Deviation is indicated as HIGH, check the following:
The positioning of the rod source, especially for the Probe detector.
Ensure that there are no other sources (including dosed patients) in the area.
Select the Setup button or press Alt+T at the bottom of the Constancy Test screen.
The Calibrated Activity and Calibration Date should match your Cs137 standard.
If a problem is found with any of the three items above, then correct the problem and
repeat the Constancy Test.
 Loading...
Loading...