Regulatory
2-56 Hardware Description
Regulatory
Philips
Software
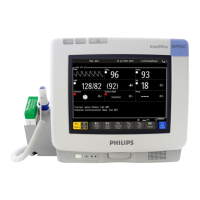
The M3290A Information Center Release E.01 software complies with applicable portions of
ANSI/AAMI EC-13 and with requirements of the Council Directive 93/42/EEC of 14 June
1993 concerning medical devices. It carries CE-marking to the European Medical Device
Directive.
Rx ONLY
Philips
Hardware
The PC workstation, Server, Philips 2-Channel Recorder, HP LaserJet printer, UPS, and
displays comply with IEC 60950, CISPR 22 Level A, and EN 50082-1. They carry CE-
marking to the European Low Voltage and EMC Directives, except for the Philips 2-Channel
Recorder, which carries CE-marking to Council Directive 93/42/EEC of 14 June 1993.
Warning Information Center system components are not suitable for installation in the Patient
Care Vicinity (Patient Environment) -- any area within 1.5 meters (4.9 ft.) horizontally
and 2.5 m (8.2 ft.) vertically above the floor from any patient care location in which
medical diagnosis, monitoring, or treatment of the patient is carried out.
0123

 Loading...
Loading...