Technical data
432
Instructions for use Zeus Infinity Empowered SW 2.n
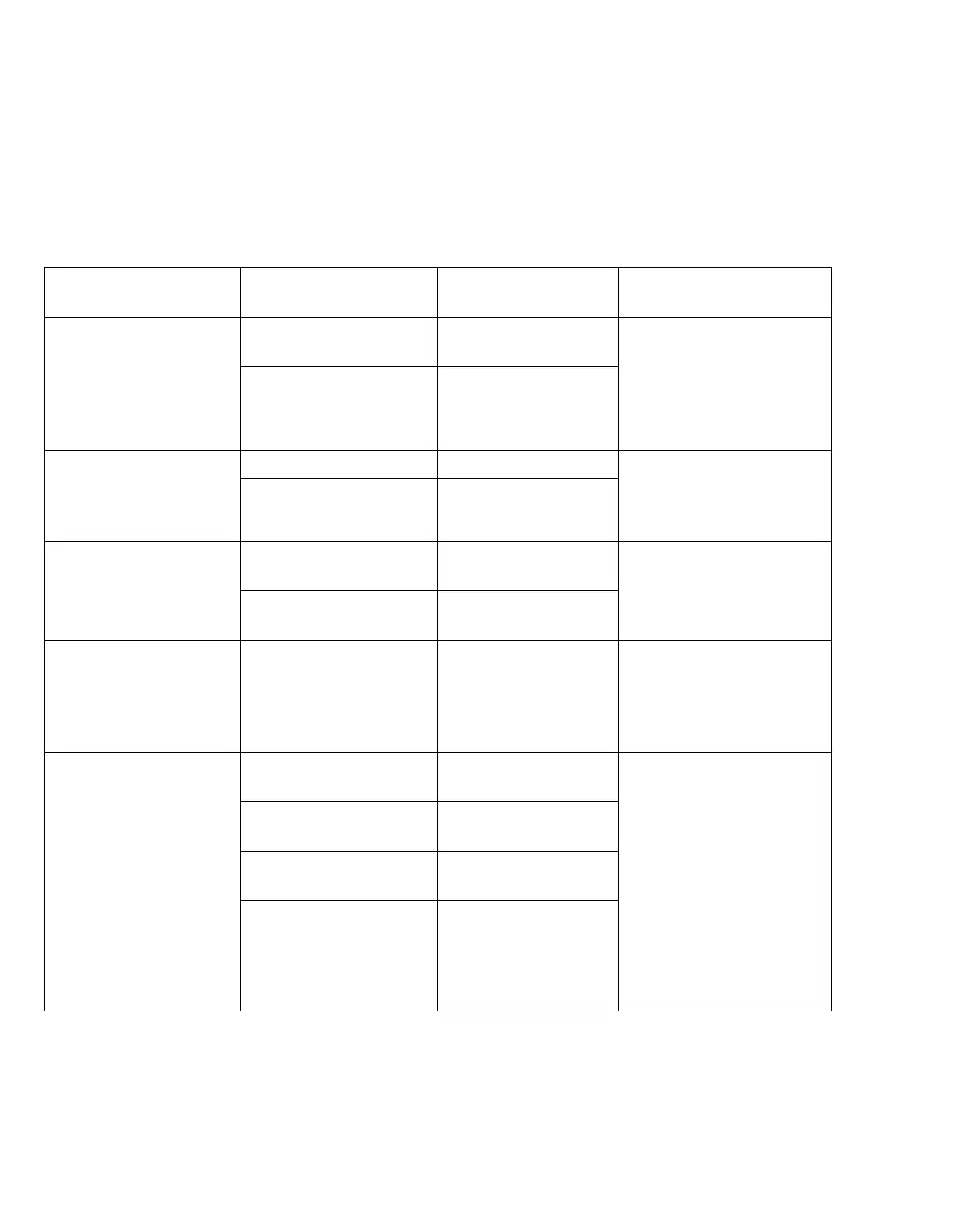
Electromagnetic immunity
The medical device is intended for use in an elec-
tromagnetic environment as specified below. The
user must ensure that the medical device is used in
such an environment.
Immunity against IEC 60601-1-2 Test
level
Compliance level of
the medical device
Electromagnetic envi-
ronment
Electrostatic discharge
(ESD) (IEC 61000-4-2)
Contact discharge:
±6 kV
±6 kV Ceramic tile and wood or
concrete flooring is prefer-
able. If floors are covered
with synthetic material, the
relative humidity should be
at least 30 %.
Air discharge: ±8 kV ±8 kV
Electrical fast tran-
sients/bursts
(IEC 61000-4-4)
Mains cables: ±2 kV ±2 kV The quality of the supply
voltage should correspond
to a typical commercial or
hospital environment.
Longer input cables /
output cables: ±1 kV
±1 kV
Surge on AC mains
lines/surges
(IEC 61000-4-5)
Common mode voltage:
±2 kV
±2 kV The quality of the supply
voltage should correspond
to a typical commercial or
hospital environment.
Differential voltage:
±1 kV
±1 kV
Power frequency mag-
netic field (50/60 Hz)
(IEC 61000-4-8)
3 A/m 3 A/m Power frequency magnetic
fields should be at levels
characteristic of a typical
commercial or hospital en-
vironment.
Voltage dips and short
interruptions on AC
mains input lines
(IEC 61000-4-11)
Dip >95 %, 0.5 periods >95 %,
0.5 periods
The quality of the supply
voltage should correspond
to a typical commercial or
hospital environment. If the
user of the medical device
requires continued opera-
tion during mains power
supply interruptions, it is
recommended that the
medical device is powered
from an uninterruptible
power supply or a battery.
Dip 60 %, 5 periods 60 %,
5 periods
Dip 30 %, 25 periods 30 %,
25 periods
Dip >95 %, 5 seconds >95 %,
5 seconds
 Loading...
Loading...