4535 611 98931 iE33 Service Manual Page 438
CSIP Level 1 Parts: Illustrations
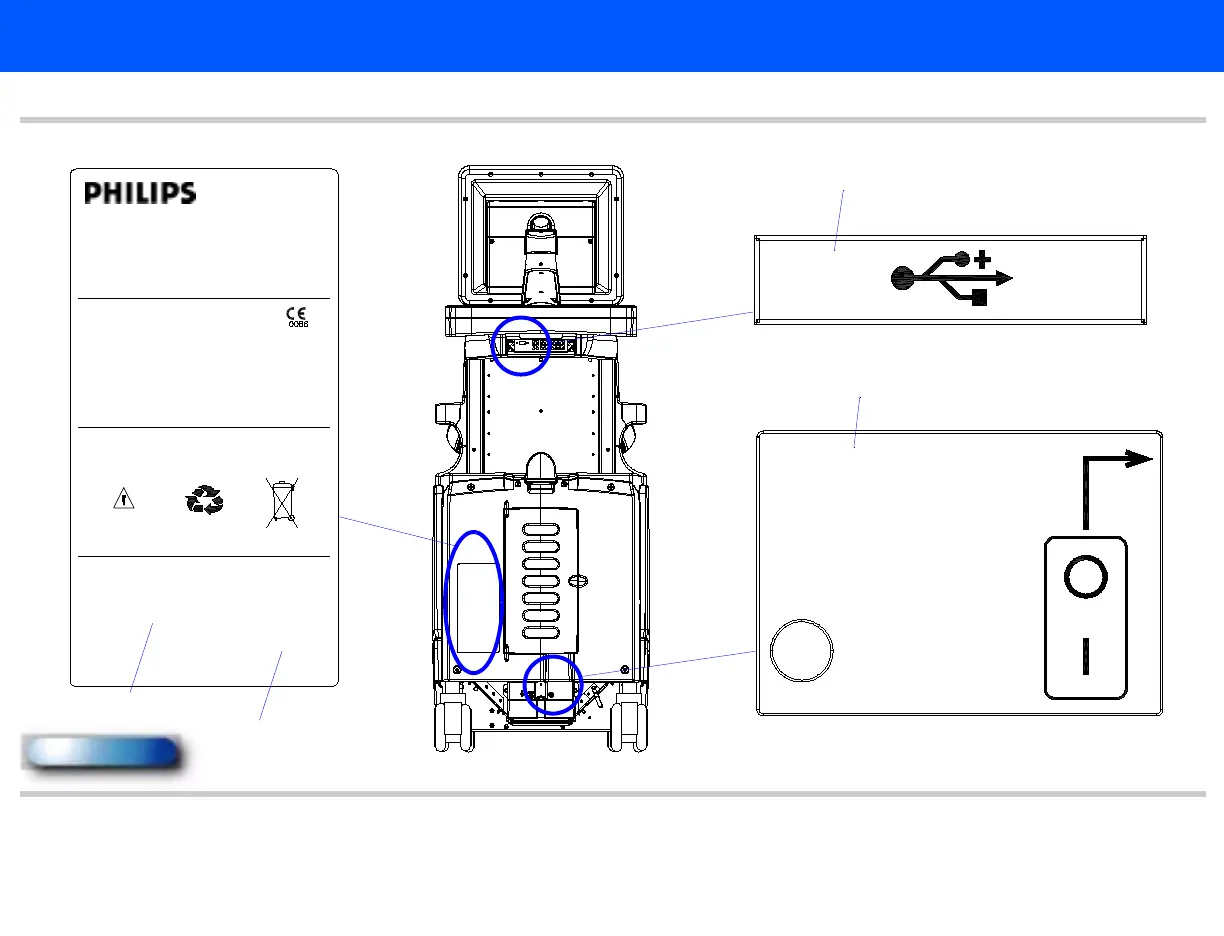
Figure 14-63 System Labeling, Rear of System (1 of 3)
S
Model No.:
Diagnostic Ultrasound System
Part No.: 8500-0064
100-120/220-240V152˜
50/60Hz, 1000VA
THIS ISM DEVICE COMPLIES WITH CANADIAN ICES-001.
CET APPAREIL ISM EST CONFORME A LA NORME NMB-001 DU CANADA.
WARN IN G: ATTACH ONLY TO EQUIPMENT DESIGNATED BY PHILIPS ULTRASOUND.
CONSULT OPERATORS MANUAL. GROUNDING RELIABILITY CAN ONLY BE ACHEIVED WHEN
THE EQUIPMENT IS CONNECTED TO AN EQUIVALENT RECEPTACLE MARKED “HOSPITAL
GRADE ONLY” OR “HOSPITAL GRADE”.
SYSTEM DISPLAY CONTAINS MERCURY; DISPOSE PROPERLY.
SYSTEM CONTAINS LEAD ACID BATTERY; DISPOSE PROPERLY.
Philips Ultrasound
Bothell WA, 98021-3003
Made in USA
453561156511
453561156521
453561161321
4535611
System
 Loading...
Loading...











